Abstract
Background/Objectives:
Most dietary interventions have metabolic effects in the short term, but long-term effects may require dietary fat changes to influence body composition and insulin action. This study assessed the effect of sustained high polyunsaturated fatty acids (PUFA) intake through walnut consumption on metabolic outcomes in type II diabetes.
Subjects/Methods:
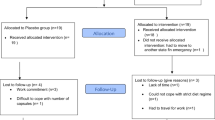
Fifty overweight adults with non-insulin-treated diabetes (mean age 54±8.7 years) were randomized to receive low-fat dietary advice ±30 g per day walnuts targeting weight maintenance (around 2000 kcal, 30% fat) for 1 year. Differences between groups were assessed by changes in anthropometric values (body weight, body fat, visceral adipose tissue) and clinical indicators of diabetes over treatment time using the general linear model.
Results:
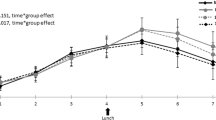
The walnut group consumed significantly more PUFA than the control (P=0.035), an outcome attributed to walnut consumption (contributing 67% dietary PUFA at 12 months). Most of the effects were seen in the first 3 months. Despite being on weight maintenance diets, both groups sustained a 1–2 kg weight loss, with no difference between groups (P=0.680). Both groups showed improvements in all clinical parameters with significant time effects (P<0.004), bar triacylglycerol levels, but these were just above normal to begin with. The walnut group produced significantly greater reductions in fasting insulin levels (P=0.046), an effect seen largely in the first 3 months.
Conclusions:
Dietary fat can be manipulated with whole foods such as walnuts, producing reductions in fasting insulin levels. Long-term effects are also apparent but subject to fluctuations in dietary intake if not of the disease process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Baecke JA, Burema J, Frijters JE (1982). A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr 36, 936–942.
Batterham MJ, Tapsell LC, Jenkins AB (2002). A comparison of bioelectrical impedance and near infra-red interactance with dual energy X-ray absorptiometry for the determination of body fat. Nutr Diet 59, 120–126.
Cao J, Schwichtenberg KA, Hanson NQ, Tsai MY (2006). Incorporation and clearance of omega-3 fatty acid in erythrocyte membranes and plasma phospholipids. Clin Chem 52, 2265–2272.
Chan JL, Heist K, De Paoli AM, Veldhuis D, Mantzoros CS (2003). The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J Clin Invest 111, 1409–1421.
Chan Jl, Mantzoros CS (2005). Role of leptin in energy deprivation states: normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 366, 74–85.
Clarke S (2001). Polyunsaturated fatty acid regulation of gene transcription: a molecular mechanism to improve the metabolic syndrome. J Nutr 131, 1129–1132.
Compher C, Hise M, Sternberg A, Kinosian BP (2005). Comparison between Medgem and Deltatrac resting metabolic rate measurements. Eur J Clin Nutr 59, 1136–1141.
Fowler MJ (2007). Diabetes treatment: part 1: diet and exercise. Clinical Diabetes 25, 105–110.
Gillen LS, Tapsell LC (2005). Structured dietary advice incorporating walnuts achieves optimal fat and energy balance in patients with type 2 diabetes mellitus. J Am Diet Assoc 105, 1087–1096.
Jacobs DR, Tapsell LC (2007). Food the fundamental unit in nutrition. Nutr Rev 65, 439–450.
Martin GS, Tapsell LC, Denmeade S, Batterham MJ (2003). Relative validity of a diet history interview in an intervention trial manipulating dietary fat in the management of type 2 diabetes mellitus. Prev Med 36, 420–428.
Matsuzaka T, Shimano H, Yagahi N, Kato T, Atsumi A, Yamamoto T et al. (2007). Crucial role of a long chain fatty acid elongase, Elov16, in obesity-induced insulin resistance. Nat Med 13, 1193–1202.
Matthews D, Hosker J, Rudenski A, Naylor B, Treacher D, Turner R (1985). Homeostasis model assessment: insulin resistance and B-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 4412–4419.
Melanson EL, Coelho LB, Tran ZV, Haugen HA, Kearney JT, Hill JO (2004). Validation of the BodyGem hand-held calorimeter. Int J Obes Relat Metab Disord 28, 1479–1484.
Morton GJ (2007). Hypothalamic leptin regulation of energy homeostasis and glucose metabolism. J Physiol 583, 437–443.
National Health and Medical Research Council (2003). Clinical Guidelines for Weight Control and Obesity Management in Adults. Commonwealth of Australia: Canberra.
Panigiagua J, Gallego de la Sacristana A, Romero I, Vidal-Puig A, Later J, Sanchez J et al. (2007). MUFA-rich diet prevents central fat distribution and decreases post-prandial adiponectin expression induced by a carbohydrate rich diet in insulin resistant subjects. Diabetes Care 30, 1717–1723.
Pirozzo S, Summerbell C, Cameron C, Glasziou P (2008). Advice on low-fat diets for obesity. Cochrane Database Syst Rev 2 Accession 12076496.
Redman LM, Heilbronn LK, Martin CK, Alfonso A, Smith SR, Ravussin E (2007). Effect of calorie restriction with or without exercise on body composition and fat distribution. J Clin Endocrin Metab 92, 865–872.
Rubenbauer JR, Johannsen DL, Baier SM, Litchfield R, Flakoll PJ (2006). The use of a handheld calorimetry unit to estimate energy expenditure during different physiological conditions. J Parenter Enteral Nutr 30, 246–250.
Sabate J (2003). Nut consumption and body weight. Am J Clin Nutr 78, 647S–650S.
Summers LK, Fielding BA, Bradshaw HA, Ilic V, Beysen C, Clark ML et al. (2002). Substituting dietary saturated fat with polyunsaturated fat changes abdominal fat distribution and improves insulin sensitivity. Diabetologia 45, 369–377.
Tapsell LC, Gillen LJ, Patch CS, Batterham MJ, Owen A, Bare M et al. (2004). Including walnuts in a low fat/modified fat diet improves HDL: total-C in patients with type 2 diabetes mellitus. Diabetes Care 27, 2777–2783.
Wang H, Storlien LH, Huang X-F (2002). Effects of dietary fat types on body fatness, leptin, and ARC leptin receptor, NPY, and AgRP mRNA expression. Am J Physiol Endocrinol Metab 282, E1352–E1359.
Wing RR, Phelan S (2005). Long-term weight loss maintenance. Am J Clin Nutr 82 (Suppl), 222S–225S.
Acknowledgements
Funding for this research was provided by the California Walnut Commission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tapsell, L., Batterham, M., Teuss, G. et al. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr 63, 1008–1015 (2009). https://doi.org/10.1038/ejcn.2009.19
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2009.19
Keywords
This article is cited by
-
Determination of Unsaturated Fatty Acids Composition in Walnut (Juglans regia L.) Oil Using NMR Spectroscopy
Food Analytical Methods (2022)
-
Biomarkers of food intake for nuts and vegetable oils: an extensive literature search
Genes & Nutrition (2019)
-
Heavy Metal Levels and Mineral Nutrient Status of Natural Walnut (Juglans regia L.) Populations in Kyrgyzstan: Nutritional Values of Kernels
Biological Trace Element Research (2019)
-
Nut consumption and risk of metabolic syndrome and overweight/obesity: a meta-analysis of prospective cohort studies and randomized trials
Nutrition & Metabolism (2018)
-
Walnut: past and future of genetic improvement
Tree Genetics & Genomes (2018)