Abstract
Objective: To assess the efficacy and safety of a low calorie soy-based meal replacement program for the treatment of obesity.
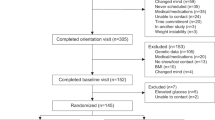
Design: A 12-week prospective randomized controlled clinical trial.
Setting: Outpatient weight control research unit.
Subjects: One hundred obese (28<BMI≤41 kg/m2) volunteers between the ages of 35 and 65 y. Seventy-four participants completed the trial.
Interventions: Participants were randomized to either the meal replacement treatment group (n=50; 240 g/day, 1200 kcal/day) or control group (n=50). Both groups at baseline received a single dietary counseling session and a pamphlet describing weight loss practices.
Main outcome measures: Weight, body fat, serum lipid concentrations.
Results: By intent-to-treat analysis, the treatment group lost significantly more weight than the control group (7.00 vs 2.90 kg; P<0.001) and had a greater change in total (22.5 vs 6.8 mg/dl; P=0.013) and LDL cholesterol (21.2 vs 7.1 mg/dl; P<0.009). Among completers only, the treatment group again lost more weight (7.1 kg; n=37 vs 2.9 kg; n=37; P=0.0001) and had a greater reduction in total cholesterol (26.1 mg/dl; n=37 vs 6.7 mg/dl; P=0.0012) and a greater change in LDL cholesterol (21.6 vs 5.5 mg/dl; P=0.0025). (For any given degree of weight loss, the reduction in LDL cholesterol was significantly greater in the treatment group.) Treatment was well tolerated and no serious side effects were detected.
Conclusions: Use of this soy-based meal replacement formula was effective in lowering body weight, fat mass and in reducing LDL cholesterol beyond what could be expected given the weight lost.
Sponsorship: This research was funded by Nutripharma. Dr Allison is a member of the United Soybean Board's Scientific Advisory Panel and Chair of the Research Grants Committee.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Alfieri, MA, Pomerleau, J, Grace, DM & Anderson, L (1995). Fiber intake of normal weight, moderately obese and severely obese subjects. Obes. Res., 3, 541–547.
Allison, DB & Pi-Sunyer, FX (1995). Obesity treatment: examining the premises. Endocr. Pract., 1, 353–364.
Allison, DB, Cappelleri, JC & Carpenter, KM (1997). Design and analysis of obesity treatment and prevention trials. InOverweight and Weight Management, ed. S Dalton, pp557–597, Gaithersburg, MD: ASPEN Publications
Allison, DB, Fontaine, KR, Heshka, S, Mentore, JL & Heymsfield, SB (2001). Alternative treatments for weight loss: a critical review. Crit. Revs. Food Sci. Nutr., 41, 1–28.
American Dietetic Association (1995). Exchange Lists for Weight Management, Washington, DC: ADA
Anderson, JW & Konz, EC (2001). Obesity and disease management: effects of weight loss on comorbid conditions. Obes. Res., 9, 326S–334S.
Begg, C, Cho, M, Eastwood, S, Horton, R, Moher, D, Olkin, I, Pitkin, R, Rennie, D, Schulz, KF, Simel, D & Stroup, DF (1996). Improving the quality of reporting of randomized controlled trials—the CONSORT statement. JAMA, 276, 637–639.
Bosello, O, Cominacini, L, Zocca, I, Garbin, U, Compri, R, Davoli, A & Brunetti, L (1988). Short and long term effects of hypocaloric diets containing proteins of different sources on plasma lipids and apolipoproteins of obese subjects. Ann. Nutr. Metab., 32, 206–214.
Bray, GA & Greenway, FL (1999). Current and potential drugs for treatment of obesity. Endocr. Rev., 20, 805–875.
Clarkson, TB (2002). Soy, soy phytoestrogens and cardiovascular disease. J. Nutr., 132, 566S–569S.
Committee for Proprietary Medicinal Products (1997). Note for Guidance on Clinical Investigation of Drugs Used in Weight Control, The European Agency for the Evaluation of Medicinal Products
Dattilo, AM & Kris-Etherton, PM (1992). Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. Am. J. Clin. Nutr., 56, 320–328.
Davidson, MH, Hauptman, J, DiGirolamo, M, Foreyt, JP, Halsted, CH & Heber, D et al (1999). Weight control and risk factor reduction in obese subjects treated for 2 years with orlistat: a randomized controlled trial. JAMA, 281, 235–242.
Derogatis, LR & Melisaratos, N (1983). The brief symptom inventory: an introductory report. Psychol. Med., 13, 595–605.
Dhurandhar, N & Allison, DB (2000). The pharmacologic treatment of obesity. Econ. Neurosci., 2, 42–52.
Eden, JA (2001). Managing the menopause: phyto-oestrogens or hormone replacement therapy?. Ann. Med., 33, 4–6.
Edlen-Nezin, L (1993). Truth or consequences: helping patients report nonadherence to medication regimens. Unpublished manuscript
Flegal, KM, Carroll, MD, Kuczmarski, RJ & Johnson, CL (1998). Overweight and obesity in the United States: prevalence and trends, 1960–1994. Int. J. Obes. Relat. Metab. Disord., 22, 39–47.
Friedman, M & Brandon, DL (2001). Nutritional and health benefits of soy proteins. J. Agric. Food Chem., 49, 1069–1086.
Frolich, ED, Grim, C & Labarthe, DR et al (1988). Recommendations for human blood pressure determination by sphygmomanometers. Report of a special task force sponsored by the Steering Committee, American Heart Association. Hypertension, 11, 210a–221a.
Hecker, HD (2001). Effects of dietary animal and soy protein on cardiovascular disease risk factors. Curr. Atheroscler. Rep., 3, 471–478.
Hermansen, K, Sondergaard, M, Hoie, L, Carstensen, M & Brock, B (2001). Beneficial effects of a soy-based dietary supplement on lipid levels and cardiovascular risk markers in type 2 diabetic subjects. Diabetes Care, 2, 228–233.
Heymsfield, SB, Wang, Z, Visser, M, Gallagher, D & Pierson, RN Jr (1996). Techniques used in the measurement of body composition: an overview with emphasis on bioelectrical impedance analysis. Am. J. Clin. Nutr., 64, (3 Suppl) 478S–484S.
Hoie, LH & Bruusgaard, D (1995). Compliance, clinical effects, and factors predicting weight reduction during a very low calorie regimen. Scand. J. Prim. Health Care, 13, 13–20.
Hoie, LH, Bruusgaard, D & Thom, E (1993). Reduction in body mass and change in body composition on a very low calorie diet. Int. J. Obes. Relat. Metab. Disord., 17, 17–20.
Kalachnik, JE (1985). Medication monitoring procedures: thou shall, here's how. InPharmacotherapy and Mental Retardation, ed. KD Gadow & AG Poling, pp244–245, Boston, MA: College-Hill Press
Kissileff, HR (1988). Satiating efficiency and the satieties. Appetite, 11, 48–53.
Lachin, JM (2000). Statistical considerations in the intent-to-treat principle. Control Clin. Trials, 21, 167–189.
Lavigne, C, Marette, A & Jacques, H (2000). Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab., 278, E491–500.
Lichtenstein, A (1998). Soy protein, isoflavones and cardiovascular disease risk. J. Nutr., 128, 1589
Lohman, TG, Roche, AF & Martorell, R (1988). Anthropometric Standardization Reference Manual, Champaign, IL: Human Kinetics Books
Peterson, L, Homer, AL & Wonderlich, SA (1982). The integrity of independent variables in behavior analysis. J. App. Behav. Anal., 15, 477–492.
Potter, SM, Baum, JA, Teng, H, Stillman, RJ, Shay, NF & Erdman, JW Jr (1998). Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am. J. Clin. Nutr., 68, (6 Suppl) 1375S–1379S.
Schafer, JL (1997). Analysis of Incomplete Multivariate Data, London, UK: Chapman & Hall
Tchernof, A, Calles-Escandon, J, Sites, CK & Poehlman, ET (1998). Menopause, central body fatness, and insulin resistance: effects of hormone-replacement therapy. Coronary Art. Dis., 9, 503–511.
Toshiaki, A & Kensuke, F et al (2000). Soy protein isolate and its hydrolysate reduce body fat of dietary obese rats and genetically obese mice. Nutrition, 16, 349–354.
Tsourounis, C (2001). Clinical effects of phytoestrogens. Clin. Obstet. Gynecol., 44, 836–842.
Westerterp-Plantenga, MS, Rolland, V, Wilson, SA & Westerterp, KR (1999). Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs high fat diets measured in a respiration chamber. Eur. J. Clin. Nutr., 53, 495–502.
Author information
Authors and Affiliations
Contributions
Guarantor: DB Allison.
Contributors: DBA was involved in the design, oversight, analysis, and the drafting of the manuscript. GG assisted in the data analysis and the drafting of the manuscript. LGS and RM were involved in the execution of the study and data interpretation. JLK conducted preliminary data analysis and assisted in the drafting of the manuscript. KRF assisted with manuscript preparation, the interpretation of the data, and editing. SH and SBH were involved in the design and oversight of the study, as well as editing drafts of the manuscript.
Corresponding author
Rights and permissions
About this article
Cite this article
Allison, D., Gadbury, G., Schwartz, L. et al. A novel soy-based meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. Eur J Clin Nutr 57, 514–522 (2003). https://doi.org/10.1038/sj.ejcn.1601587
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ejcn.1601587
Keywords
This article is cited by
-
Impact of Partial Meal Replacement on Glycemic Levels and Body Weight in Indian Patients with Type 2 Diabetes (PRIDE): A Randomized Controlled Study
Diabetes Therapy (2022)
-
Study Protocol for the Effects of Formula Diet with Dapagliflozin on Metabolic Improvement and Body Composition in Type 2 Diabetes Mellitus
Diabetes Therapy (2019)
-
Effect of soy on metabolic syndrome and cardiovascular risk factors: a randomized controlled trial
European Journal of Nutrition (2018)
-
Korean traditional Chungkookjang improves body composition, lipid profiles and atherogenic indices in overweight/obese subjects: a double-blind, randomized, crossover, placebo-controlled clinical trial
European Journal of Clinical Nutrition (2016)
-
Effect of protein source during weight loss on body composition, cardiometabolic risk and physical performance in abdominally obese, older adults: A pilot feeding study
The Journal of nutrition, health and aging (2015)