Abstract
Adenovirus and adeno-associated virus vector-mediated gene delivery is limited by the induction of a humoral immune response that prevents readministration. To determine whether viral delivery in the “preimmune” fetus would produce dose- or time-dependent tolerance, we evaluated the humoral immune response after sequential pre- and postnatal virus administration. We evaluated six injection route and viral dose combinations of adenovirus (intra-amniotic, intrahepatic, and intramuscular injection at 4 × 108 and 2 × 109 particles/fetus) at d 15 postconception (p.c.); three route and dose combinations at d 13 p.c. (intramuscular injection at 1 × 108, 3 × 108, and 5 × 108 particles/fetus); and one route and dose combination of adeno-associated virus (intramuscular at 2.5 × 1010 genome copies/fetus) at d 15 p.c. In utero injection of either viral vector at any route and dose combination resulted in the production of low titers of neutralizing antivirus and antitransgene (β-galactosidase) antibodies. This primary immune response only partially blocked transgene expression after the readministration of viral vectors postnatally. However, delivery of the virus postnatally triggered an immune response that completely blocked transgene expression after a third viral injection. Together, these results suggest that, for B6/129 F1 mice, in utero injection of recombinant adenovirus or adeno-associated virus between d 13 and 15 p.c. does not induce tolerance to the viral vector or transgene product.
Similar content being viewed by others
Main
The potential of prenatal gene therapy to treat severe genetic and developmental disorders has sparked new research and debate as to its feasibility and reliability. The feasibility of in utero gene transfer has been demonstrated in a number of animal models, including mouse, rat, rabbit, and sheep, using recombinant Ad (1–5) and recombinant AAV (5, 6) vectors. The potential success of fetal gene therapy will depend on long-term transgene expression that begins early in fetal life and continues at therapeutic levels into postnatal life. If the transgene cannot replicate itself during cell division or loses its expression over time, readministration may be necessary. However, readministration of the same vector may not be effective because of the induction of host humoral immune responses (7–9). It has been speculated that immunologic immaturity of the fetuses may reduce the immune response or allow the induction of immune tolerance to the virus (10, 11).
Another consideration is that tolerance is essential to prevent an anaphylactic response in the case of the recipient who could recognize the therapeutic transgene product as a foreign protein. For example, in the case of the X-linked inborn error of urea synthesis, ornithine transcarbamylase deficiency, homozygous males do not produce any of this enzyme (12). Introduction of a virus carrying the gene coding for this protein could potentially precipitate a massive immune response unless the host recognized the protein as “self.” If tolerance to a transgene protein is indeed possible, correction of several genetic defects may be feasible. On the other hand, tolerance to a viral vector, such as an Ad, could lead to susceptibility to a potentially life-threatening infection from this normally rather benign virus.
Although the immune response to Ad or AAV vectors has not been studied in human fetuses and neonates, it is generally believed that inflammatory and immune responses in the human are not fully developed before birth (10). Studies suggest that there is a window of opportunity for transplanting HSC before 18–20 wk of gestation (13, 14). These studies have led to the general impression that foreign substances can be administered safely and will be tolerated up to this point in gestation. It is not clear, however, how to establish tolerance in fetuses; and it remains to be determined whether there is a specific time when self/nonself recognition is established.
The immune response to Ad vectors after in utero administration has been investigated in a number of animal models. Sekhon and Larson (2) reported that, in rats, exposure to foreign antigens before the maturation of thymic processing of lymphocytes leads to tolerance to these antigens. However, others have reported inflammatory responses to virus-transduced cells during fetal life. For example, pulmonary inflammation was observed in fetal sheep after intratracheal and intra-amniotic Ad administration (4, 15), as was the development of anti-Ad antibodies (3, 16). Possible explanations for these conflicting results may be the route and dose of viral administration, or the different animal species being used.
The present study focuses on the humoral immune response to recombinant Ad and AAV vectors after in utero injection and subsequent postnatal readministration. It provides support for the hypothesis that there is induction of an immune response and an absence of tolerance after in utero gene transfer.
MATERIALS AND METHODS
Mice.
Adult B6/129F1 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, U.S.A.) and were housed in a specific pathogen-free animal facility at the Children's National Medical Center. All animal experiments were carried out according to institutional guidelines for animal use and the protocols were approved by the Institutional Animal Care and Use Committee.
Preparation of Ad and AAV vectors.
The E1-deleted recombinant Ad carrying either the Escherichia coli lacZ gene encoding β-galactosidase (β-gal) (designated Ad.CBlacZ) or human growth hormone (Ad.CMVhGH) was generated as previously described (17). LacZ gene expression was controlled by the cytomegalovirus (CMV) enhancer and chicken β-actin gene promoter (CB promoter); hGH was under the control of a CMV enhancer/promoter. Viral preparation was performed as previously described (18). Viruses were replicated in HEK 293 cells, and cell lysates were aliquoted and stored at −80°C. Viruses were purified from a frozen cell lysate on the day of the injection by two rounds of CsCl density gradient centrifugation and were then desalted on Bio-Gel P-6 desalting column (Bio-Rad Laboratories, Hercules, CA, U.S.A.) with PBS. The titer of the virus preparation was determined both by spectrophotometric absorbency at 260 nm and by plaque assay (19). The particle to plaque forming unit ratio was <100. Purified viruses were suspended in PBS at the desired concentrations.
Recombinant AAV vectors containing either the β-gal gene under the control of CMV promoter (AAV.CMVlacZ) or hGH gene (AAV.CMVhGH) were produced as described by Xiao et al.(20). Briefly, the cis plasmid (with AAV inverted terminal repeats), the trans plasmid (with the AAV rep and cap gene), and a helper plasmid (pFΔ6, which contains an essential region from the Ad genome) were co-transfected into HEK 293 cells at a ratio of 1:1:2 by calcium phosphate precipitation in an Ad-free system. The cells were harvested 96 h later. The AAV was purified through two rounds of CsCl density gradient centrifugation, desalted by dialysis at 4°C against PBS, aliquoted, and kept at 4°C. The titer was determined by quantitative dot blot hybridization. The purity of the AAV vector preparation was addressed by colloidal brilliant blue G staining (Sigma Chemical, St. Louis, MO, U.S.A.) and Western blotting of viral proteins separated by SDS-PAGE. The same lot of AAV virus was used for all experiments.
In utero injection.
To generate fetuses for in utero injection, 10- to 12-wk-old female B6/129F1 mice were mated with B6/129F1 males for one night and checked for vaginal plugs the next morning. Mice bearing the plug were housed in individual cages, and the day that the plug was found was counted as d 0 p.c. On the day of injection, the pregnant female mouse (d 15 p.c. or d 13 p.c.) was anesthetized with Metofane (Pitman-Moore, Mundelein, IL, U.S.A.). A laparotomy was performed and the uterus was gently eviscerated with a pair of cotton-tipped applicators. Injection of fetuses was performed with a glass micropipette needle (diameter: 50–100 μm), pulled with a P-30 needle puller and polished with a BV-10 beveler (both made by Sutter Instrument Co., Novato, CA, U.S.A.), through the uterine wall into the amniotic cavity, the liver, or the front limb of the fetus. Five microliters of virus was delivered by injection at d 15 p.c. and 2 μL by injection at d 13 p.c. Control fetuses were injected with 5 μL of PBS into the same locations. The uterus was returned to the abdominal cavity and the abdominal wall was then closed with sutures. The mouse was kept on a warm pad and returned to its cage after recovery.
Determination of β-gal activity in tissue samples.
Frozen sections (10 μm) of tissue were fixed in 0.5% glutaraldehyde at room temperature for 10 min, washed with PBS, and stained for 2 h at 37°C in PBS containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mM MgCl2, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal). The sections were then counter-stained with hematoxylin and mounted for microscopic evaluation.
Determination of anti-Ad Nab titer.
Anti-Ad Nab titer was analyzed by assessing the ability of serum antibody to inhibit transduction of the reporter virus Ad.CBlacZ into A549 cells. To determine the Nab activity, plasma samples were diluted in F-12 K nutrient mixture (Kaighn's modification; Invitrogen, Carlsbad, CA, U.S.A.) containing 2% fetal bovine serum (FBS) in 2-fold steps starting from 1:4. Each antibody dilution (50 μL) was mixed with 50 μL of F-12 K containing 2% FBS and 1 × 105 pfu of Ad.CBlacZ, incubated for 1 h at 37°C, and applied to 80% confluent A549 cells in 96-well plates. The cells were incubated at 37°C for 24 h, fixed in 0.5% glutaraldehyde at room temperature for 10 min, washed with PBS, and stained for 2 h at 37°C in PBS containing 5 mM K3Fe(CN)6, 5 mM K4Fe(CN)6, 1 mM MgCl2, and 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-galacto-pyranoside (X-gal). The titer of Nab was defined as the highest dilution at which <50% of the cells stained positive for lacZ. A column-purified mouse anti-Ad Nab was used in the experiments as a standard. The purified antibody (1 mg/mL) had a neutralizing titer of 3200. The sensitivity of the assay was 0.2 ± 0.1 μg/mL using the purified Nab. The assay was linear within a range of 0.1–2 μg/mL using the same antibody.
Determination of anti-AAV Nab titer.
To determine the anti-AAV Nab activity, plasma samples were diluted in Dulbecco's modification of Eagle's medium (DMEM, Fisher Scientific, Pittsburgh, PA, U.S.A.) containing 2% FBS (JRH Biosciences, Lenexa, KS, U.S.A.) in 2-fold steps starting at 1:4. Each antibody dilution (50 μL) was mixed with 50 μL of DMEM containing 2% FBS and 1 × 105 particles of AAV.CMVlacZ, incubated for 1 h at 37°C, and applied to 80% confluent 84–31 cells in 96-well plates. The cells were incubated at 37°C for 24 h, fixed in 0.5% glutaraldehyde at room temperature for 10 min, washed with PBS, and stained as described above.
Determination of anti-β-gal antibody titer.
Ninety-six-well high-binding ELISA plates (Costar, Cambridge, MA, U.S.A.) were coated with 100 μL of E. coli β-gal (Sigma Chemical, 1 μg of protein per well) in PBS overnight at room temperature, washed four times in PBS/0.1% Tween 20, and incubated in PBS/1% BSA for 1 h at room temperature. Appropriately diluted serum samples (100 μL) were added to plates and incubated overnight at room temperature. Plates were washed four times with PBS/0.1% Tween 20 and incubated with peroxidase conjugated goat anti-mouse anti-bodies (1:1000 dilution) for 2 h at room temperatue. Plates were washed as above, and peroxidase substrate (Kirkegaard & Perry Laboratories, Gaithersburg, MD, U.S.A.) was added. Optical density was read at 400 nm on a microplate reader. A mouse anti-β-gal antibody (Sigma Chemical) was used for standard curve.
Determination of plasma hGH concentration.
Plasma hGH concentration was determined using a hGH ELISA kit (Rocher, NJ, U.S.A.) according to manufacture instructions.
RESULTS
Humoral immune response to Ad.CBlacZ after d 15 p.c. injection.
In a preliminary experiment, we injected d 15 p.c. mouse fetuses in the front limb with 2 × 109 particles of recombinant Ad carrying the gene encoding hGH. Mice born after the injection were bled at a number of time points, and the levels of serum hGH were measured by ELISA assay. HGH expression was evident 2 wk after birth and was still detectable 7 mo after birth (Fig. 1). A second injection of Ad carrying the lacZ gene encoding β-gal (Ad.CBlacZ) 3 wk after birth also resulted in significant lacZ expression in the liver (Fig. 2A), comparable to that in a naïve animal (Fig. 2B). This result led to several possible explanations: (1) the in utero injection induced tolerance to the virus and to the transgene product; (2) the in utero injection led to unresponsiveness to the virus, i.e. the virus was ignored by the immature immune system, but it did not induce tolerance; or (3) the in utero injection resulted in a weak primary immune response that did not block infection or transgene expression after readministration of the virus.
Ad-mediated transgene expression after Ad readministration. B6/129F1 mouse fetuses were injected i.m. at d 15 p.c. with 1 × 109 particles of Ad.CMVhGH. The animals were reinjected i.v. with 5 × 1010 particles of Ad.CBlacZ on d 21 after birth. (A) β-galactosidase staining indicative of lacZ expression (blue) in the liver, 4 wk after the second viral injection. (B) β-galactosidase staining (blue) in the liver of control mice that did not receive in utero injection, but were injected and killed at the same time as mice in (A). Representative pictures are shown.
To investigate these three possibilities, we systematically evaluated the humoral immune response to the virus and transgene product after in utero injection in B6/129F1 mice. Considering that different routes and doses of viral administration could trigger different types of immune responses, we tested three routes of injection (i.m., i.h., and i.a.) using two viral doses, 2 × 109 particles/fetus (high dose) and 4 × 108 particles/fetus (low dose). As depicted in Figure 3A, mouse fetuses were first injected with Ad.CBlacZ in utero at d 15 p.c. At least three fetuses were injected with each dose/route combination, with the exception that only the high dose was used for the i.a. route. The i.a./low dose combination resulted in no detectable transgene expression in the fetus and was therefore deleted from the study. Control fetuses were injected with PBS. Blood samples were taken at d 14 after birth, and anti-Ad Nab levels were determined. On d 15, the animals were injected i.v. with a second virus carrying the hGH gene (Ad.CMVhGH, 5 × 1010 particles/mouse). Blood samples were collected on a weekly basis after the second injection to determine the levels of hGH and anti-Ad Nab. On d 36, a third viral injection was given by either i.v. (Ad.CBlacZ, 1 × 1011 particles/mouse) or i.m. (Ad.CBlacZ, 2 × 1010 particles/mouse) route. The animals were killed on d 42 and examined for lacZ expression. Blood samples were also collected and analyzed for anti-Ad Nab and anti-β-gal antibody levels.
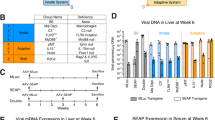
Experimental design for in utero Ad (A) and AAV (B) injections. The route and dose of injection are shown below the line. The date of injection and testing are shown above the line. d, day;p, particles;GC, genome copy;Nab, neutralizing antibody; β-gal, beta-galactosidase;i.m., intramuscular;r.o., retro-orbital.
The rationale behind this experimental design was as follows. Based on our initial observation in fetally injected mice, the first viral injection at d 15 p.c. would not block the transgene expression from the second viral injection (at d 15 after birth). Depending on the immune status of the animal after the first viral injection, however, the second viral injection would (if the animal was not tolerant to the virus) or would not (if the animal was tolerant to the virus) induce a humoral immune response. The immune status is reflected by the effectiveness of the third viral injection and by the Ad- or β-gal-specific antibody levels after the second and third viral injections. Viruses carrying hGH were used for the second injection so that transgene (lacZ) expression from the third viral injection could be easily identified.
As shown in Table 1, in utero injection using all route/dose combinations led to low levels of anti-Ad Nab at d 14 after birth. The specific titer of antibody could not be determined because it was out of the sensitivity range of the Nab assay. However, when plasma samples from the same groups of animals were pooled and assayed, some Nab activity was detected at the lowest dilution (1:4). The second viral injection (Ad.CMVhGH, 5 × 1010 particles/mouse, i.v.) was given on d 15, and the level of transgene expression was monitored by measuring the plasma hGH concentration. As shown in Figure 4, it appeared that the preexisting Nab partially blocked transgene expression after the second viral injection, as evidenced by the lower hGH levels in fetally injected animals compared with controls. After the second viral injection, all animals developed high titers of anti-Ad Nab by d 35 after birth (3 wk after the second viral injection, Table 1). The third viral injection was given at d 36 (Ad.CBlacZ, 1 × 1011 particle/mouse) by either i.v. or i.m. route. Whereas no transgene expression was detected in animals receiving an i.v. injection (data not shown), lacZ expression was detected in animals receiving an i.m. injection on d 42 (Fig. 5). In addition, there was clear indication of lymphocyte infiltration at the injection site. The anti-Ad Nab titers were further elevated in all animal groups after the third viral injection (Table 1).
Transgene expression from the second Ad injection in mice receiving an in utero injection at d 15 p.c. B6/129F1 mouse fetuses were injected at d 15 p.c. with Ad.CBlacZ by the route/dose combination indicated. The animals were reinjected with Ad.CMVhGH (5 × 1010 particle/mouse, r.o.) at d 15 after birth and the plasma hGH level was analyzed 6 d later. High dose: 2 × 109 particles/fetus; low dose: 5 × 108 particles/fetus. Control animals were injected with PBS at d 15 p.c. following Ad viral injection. Three mice were used for each route/dose combination. Values represent mean ± SD.
Transgene expression and lymphocyte tissue infiltration after a third Ad injection in mice receiving an in utero injection at d 15 p.c. β-galactosidase staining indicative of LacZ expression (blue) in muscles is shown 6 d after the third intramuscular injection with Ad.CBlacZ (2 × 1010 particles/mouse). Lymphocyte infiltration is indicated by the arrow.
We next evaluated the level of antibody to β-gal. It should be noted that, although the experimental animals received a total of three viral injections (d 15 p.c., and d 15 and 36 after birth), only the first and last virus contained the lacZ gene. The first assay for anti-β-gal antibody was carried out using plasma samples taken on d 35 after birth, before the third viral injection. The assay was repeated on d 42, 6 d after the third viral injection (the second challenge of β-gal). As shown in Table 1, all animals produced detectable levels of anti-β-gal antibody after the in utero injection. The antibody titer increased significantly after restimulation in the i.m./low-dose, i.m./high-dose, and i.h./high-dose groups, but remained unchanged in the i.h./low-dose and i.a./high-dose groups (Table 1).
Humoral immune response to Ad virus after in utero administration at d 13 p.c.
To determine whether in utero injection at an earlier gestational age leads to a different immune response, we injected fetuses at d 13 p.c. with Ad.CBlacZ. Our initial experiment showed that i.a. and i.h. injections invariably led to high mortality rates (>80% with PBS only) in fetuses. The only feasible route of viral delivery was by i.m. injection. This resulted in a survival rate of about 70% at doses ranging from 1 × 108 to 5 × 108 particles/fetus; the survival rate, however, decreased rapidly at doses higher than 5 × 108 particles/fetus.
We repeated the experiment depicted in Figure 3A with i.m. injections of Ad.CBlacZ at d 13 p.c. using three viral doses (1 × 108, 3 × 108, or 5 × 108 particles/fetus). As shown in Table 2, anti-Ad Nab was detected only in mice receiving the highest viral dose at d 14 after birth. The hGH expression after the second dose of virus appeared to correlate inversely with the viral dose used for the in utero injection and, presumably, with the anti-Ad Nab levels before the second injection (Fig. 6). However, all animals developed high levels of anti-Ad Nab after the second viral injection, which was further increased by the third viral injection. Anti-β-gal antibody was detectable in all groups at d 35, and the levels of antibody appeared to correlate with the viral dose (Table 2). Similar to that observed in d 15 p.c. fetuses, the third i.v. viral injection led to no transgene expression, whereas the i.m. injection resulted in local lacZ expression and lymphocyte infiltration (data not shown).
Transgene expression after the second Ad injection in mice receiving in utero injection at d 13 p.c. B6/129F1 mouse fetuses were injected i.m. on d 13 p.c. with Ad.CBlacZ at doses indicated. The animals were reinjected with Ad.CMVhGH on d 14 after birth, and plasma hGH level was analyzed 6 d later. Control animals received PBS injection in utero. Five mice were injected with 1 × 108 particles/fetus, three mice with 3 × 108 particles/fetus, and four mice with 5 × 108 particles/fetus. Values represent mean ± SD.
Humoral immune response to AAV.CMVlacZ after in utero injection at d 15 p.c.
To evaluate immune responses to AAV vectors administered in utero, we performed experiments similar to those with the Ad vectors. As depicted in Figure 3B, mouse fetuses were injected in the front limb with recombinant AAV carrying the lacZ gene (AAV.CMVlacZ 2.5 × 1010 GC/fetus) at d 15 p.c., followed by a second injection of AAV.CMVhGH (2 × 1011 GC/mouse) in the quadriceps at d 43 after birth, and a third injection of AAV.CMVlacZ (2 × 1011 GC/mouse) in the anterior tibialis at d 79. The extended time period between injections was based on the slower rate of AAV-mediated transgene expression compared with Ad. AAV transgene expression usually requires several weeks to reach peak levels because of the rate-limiting step of second-strand viral DNA synthesis. AAV showed little toxicity at 2.5 × 1010 GC/fetus, the highest dose possible using available AAV stocks (5 × 1012 GC/mL, 5 μL per fetus). Similar to Ad vectors, the in utero injection of AAV.CMVlacZ induced production of low titers of anti-AAV Nab and anti-β-gal antibody (Table 3). A second i.m. injection of AAV.CMV hGH was also effective and led to expression of hGH for at least 2 mo (Fig. 7). The animals developed high levels of anti-AAV Nab, however, after the second injection (Table 3). This resulted in the complete neutralization of the virus after the third injection, as evidenced by the lack of lacZ expression in the anterior tibialis 1 mo later (data not shown).
Transgene expression after the second AAV injection in mice receiving in utero injection at d 15 p.c. B6/129F1 mouse fetuses (eight fetuses) were injected i.m. on d 15 p.c. with AAV.CMVlacZ (2.5 × 1010 GC/fetus). The animals were reinjected with AAV.CMVhGH (1 × 1011 GC/mouse, i.m.) on d 43 after birth, and plasma hGH level was analyzed at various timepoints. Values represent mean ± SD.
DISCUSSION
It is well documented that administration of recombinant Ad or AAV vectors induces a humoral immune response in adult mice, which renders the subsequent viral administration ineffective (7, 9). Our results and those of previous studies (5, 6, 16, 21) have demonstrated long-term transgene expression after in utero administration of Ad and AAV vectors and effective readministration of the same vector after birth. The purpose of this study was to determine whether administration of these vectors during fetal development before the maturation of the immune system could induce tolerance and/or modify the humoral immune response to the subsequent administration of the same vector.
Induction of tolerance across allogeneic barriers after in utero administration of HSC has been evaluated in a few murine models (22–25). Significant and durable engraftment of MHC-mismatched HSC has been achieved with HSC transplantation between d 11 and 13 p.c (23). Yet, although in utero administration of Ad (21) and AAV (6) vectors in mouse fetuses at d 15 p.c. has not been shown to elicit a humoral immune response or cytotoxicity, subsequent viral administration has led to antibody production. Tolerance to Ad or AAV vectors after in utero injection has not yet been reported.
Factors that may affect the outcome of in utero viral injection.
Several factors must be considered regarding the effectiveness of inducing tolerance by in utero injection of viral vectors. First, the route of administration determines which immune organs and cell populations will be involved in the response. For example, virus injected i.v. is taken up primarily by the spleen and liver; whereas i.m. administration results in the virus being directed to local lymph nodes. Differences in the lymphoid cell population of these organs may generate differences in the quality of immune response (26, 27). Second, whether or not the fetus will develop an immune response against the vector and/or transgene is likely to be influenced by the stage of fetal development when the virus is administrated. It was shown that d 12–14 p.c. is a critical period of T-cell maturation (28). On the other hand, Strasser et al.(29) reported that B-cell precursor development in the mouse fetal liver consist of two distinct phases: a stromal cell–dependent phase between d 13 and 15 p.c., and a stromal cell–independent phase after d 16 p.c.. It is still unclear whether these discrete periods of T- and B-cell development relate to the establishment of self-/nonself-recognition in mice. Third, the dose of virus and the level of viral and transgene expression may also impact on the nature and quality of the immune response (30). Finally, the nature of the immune response may be virus-specific. For example, Fields et al.(31) reported that the i.v. injection of an Ad carrying the gene for human factor IX resulted in tolerance to human factor IX in adult C57BL/6 mice. However, tolerance to factor IX was not induced by i.m. injections of the same Ad or by an AAV vector carrying the same transgene (31).
Considering the above-mentioned factors, we first injected d 15 p.c. mouse fetuses with two different doses of Ad, using three different routes of administration. The experiment was designed to distinguish between three possible outcomes:
-
1
The in utero injection would induce complete immune tolerance to both the virus and transgene product. If this were the case, the second and third viral injection would not induce an immune response. No Nab to the virus or β-gal would develop after the first, second, or third viral injection. HGH expression levels after the second injection would be similar when comparing control to experimental groups. LacZ expression after the third viral injection would also be comparable to that in control mice.
-
2
The in utero injection would lead to immune unresponsiveness to the virus, i.e. the virus would be ignored by the immature fetal immune system. In this case, the viral injection would not induce a humoral immune response in the fetus, but neither would it result in tolerance to the virus; the animal would respond to the second viral injection as if naïve. Normal hGH expression would be expected. However, the second injection would result in a primary humoral immune response and the production of Nab to the virus and/or to β-gal. These antibodies would block or partially block the transduction and transgene expression after the third viral injection. Robust antibody response would be expected after the third viral injection as a result of the secondary humoral immune response.
-
3
The in utero injection would result in a normal immune response. In this scenario, Nab to the virus and/or to β-gal would develop after the in utero injection and would block or partially block hGH expression from the second viral injection. Nab titers would increase significantly after the injection of Ad.CMVhGH as a result of a secondary immune response to the virus.
In utero injection of Ad vectors.
We found that all dose/route combinations for d 15 p.c. Ad injection led to the development of low titers of antibodies to the virus and to its transgene product. The virus-Nab activity was reflected by the lower levels of hGH expression after the second viral injection. Moreover, the robust antiviral antibody response after the second viral challenge suggests that the animals were primed by the in utero injection. It therefore appeared that injection of the Ad vector at d 15 p.c., under the tested conditions, resulted in neither tolerance nor unresponsiveness to the virus as a whole. Rather, the injection led to a primary humoral immune response to both the viral proteins and transgene product. However, without detailed analysis of the anti-Ad Nab profiles, we could not rule out the possibility that the in utero injections might have induced a partial tolerance to certain viral proteins or epitopes.
In our experiments, we used two reporter proteins: hGH, a secreted protein, and β-gal, a cytoplasmic protein. Both are soluble proteins and are known to generate humoral responses (32). It is not yet clear whether one is more efficient than the other in the induction of tolerance or immune response in a prenatal or neonatal setting. Theoretically, a cytoplasmic or membrane-bound protein may be less immunogenic than a secreted protein in antibody production, but the cytoplasmic or membrane-bound protein may attract the antibody to the cells and lead to their destruction. In our experiments, anti-hGH and anti-β-gal antibodies were detected at high titers after virus injection. Using murine cDNA as transgene may avoid the anti-transgene immune response, but it will not influence the antiviral humoral response.
The anti-Ad Nab that developed after the second viral infusion was sufficient to neutralize all i.v. injected viruses, but may not have been adequate to prevent infection of muscle fibers by a local injection of Ad. However, the massive lymphocyte infiltration at the injection site only 6 d after viral injection is a strong indication of the secondary cellular immune response that would likely have resulted in the destruction of virus-containing cells.
Because of the high mortality rates in fetuses injected at d 13 p.c. through i.a. and i.h. routes, the study focused on i.m. injection using three viral doses. All injected animals developed anti-Ad antibody that blocked transgene expression after the second viral injection in a dose-dependent manner. Taken together, these results suggest that injection of recombinant Ad at d 15 p.c. or d 13 p.c. in B6/129F1 fetuses did not induce tolerance to Ad or β-gal, nor was it ignored by the fetal immune system. Instead, the in utero injection induced a humoral immune response to both Ad and β-gal.
Considering the fact that immune responses to Ad vary significantly among mice of different genetic background (33), it is still possible that a similar treatment in a different mouse strain might have led to tolerance to viral vectors. In our study, we used hybrid B6/129F1 mice that are primarily used as controls for studies involving knockout models of a variety of human diseases. It is possible that some inbred mouse strains may be more suitable for induction of tolerance than other strains. Recently, tolerance to human factor IX after i.v. injection of Ad vector (31) and to β-gal after intrathymic injection of Ad vector (34) has been reported in adult and neonatal C57Bl/6 mice, respectively. Therefore, C57Bl/6 mice may be more sensitive to tolerance induction. This may also explain the differences observed in antibody production after Ad viral administration at d 15 p.c. in mouse fetuses, comparing our study to that of Lipshutz et al.(21). These differences, however, may also be related to the sensitivities of assays used for detection of antibody.
Because T-cell migration to the thymus starts at d 11 p.c. (28), we attempted to inject Ad before d 11 p.c. However, most of the fetuses did not survive the injection, even though the viral dose was adjusted based on the size of the fetus. Other investigators have made similar observations. Turkay et al.(1) reported that <50% of fetuses survived intraplacental injection of Ad.CMVlacZ at d 11.5 p.c. Carrier et al.(23) found that in utero stem cell transplantation at this stage led to an average survival rate of 30%. Recently, transgenic mice carrying an E1-E3 deleted Ad virus have been generated (35). It was found that the transgene conferred partial embryonic lethality, potentially because expression of Ad genes during embryonic development is lethal. Furthermore, transgenesis for Ad did not decrease the immune response after postnatal administration. It is possible, therefore, that immune tolerance to Ad cannot be induced by in utero viral administration.
The concept that the neonatal immune system is predisposed to tolerance rather than to immune response has recently been reexamined by Matzinger and colleagues (36), who showed that both neonatal and adult T cells can be immunized or tolerated depending on the number of antigen-presenting cells. Our results support the “danger theory of tolerance,” which proposes that foreign antigens elicit an immune response if they are dangerous to the host (37). Another possibility is that failure to induce classical tolerance could be attributed to the inability of the antigen to reach the thymus after Ad injection. Recently, induction of stable prenatal tolerance to β-gal was achieved by in utero infection of thymic epithelial cells with a retroviral vector carrying the lacZ gene in preimmune sheep fetus (38).
In utero injection of AAV vectors.
AAV vector elicits weaker immune responses and can result in long-term gene transfer and expression (39). It thus appears to be a better candidate for tolerance induction since the existing theory concerning the induction of stable prenatal tolerance requires early prenatal antigen exposure to induce tolerance and persistence of the antigen to maintain tolerance throughout life. In our experiments, AAV vector led to stable, long-term expression of the transgene after both the first in utero and the second postnatal injections. However, all animals developed low titers of neutralizing anti-AAV antibody after the first in utero injection, but the titer was insufficient to completely block the entrance of the virus after the second postnatal injection. Unlike the E1-deleted recombinant Ad, which still contains a large number of viral genes and may synthesize some viral proteins in the infected cells, recombinant AAV contains no viral genes and the viral capsid proteins are the only targets for an immune response. Despite this, the fetal immune system appeared to respond to these proteins rather efficiently.
A third AAV injection of AAV.lacZ did not result in any detectable β-gal expression in the anterior tibialis muscle group that was different from muscle used for the first and second injections. In other experiments (data not shown), we also failed to detect hGH expression after a third AAV.hGH injection in mice that had received two previous injections of AAV.lacZ. These results suggest that the level of neutralizing anti-AAV antibody after the second injection was high enough to completely prevent viral entry after the third AAV injection under the conditions used in our experiment. However, it is possible that different results could be obtained by modulating the experimental conditions. Furthermore, induction of tolerance/immune response may be affected by the integrating status of the viral genome. It has been shown that recombinant AAV does not integrate but rather exists in the cell in an episomal form (40). Thus, an integrating virus may be more suitable for induction of tolerance.
Avoiding immune response without the tolerance induction.
The high concentrations of viral vector needed to attain a therapeutic level of transgene expression can also boost the immune response. One approach to solving this problem is to increase gene transfer by directing viral vector entry via an alternative receptor system. Recently, some effective genetic and chemical modifications of viral capsids using different viruses have been reported (41–43). This is certainly a feasible approach to avoid the booster effect. Successful multiple viral administration has also been achieved using Ad of different serotypes (44, 45).
Transient immunosuppression with nonselective immunosuppressive drugs has been evaluated in a number of studies as a tool to prevent formation of Nab against viral vectors and to reduce the resultant cellular immune response (45, 46). Most nonselective immunosuppressive drugs are cytotoxic to proliferating cells, however, so the safety of in utero administration of such drugs is of concern. Administration of specific antibodies against co-stimulatory signals (such as anti-CD40L antibody or anti-B7.1/2 antibody) has been used to block both the humoral and cellular responses (46, 47). It has also been reported that anti-CD4 antibody given before and after Ad administration blocks antiviral Nab production and allows efficient transgene expression after second or third viral injections (48). Therefore, it is possible that in utero viral administration coupled with transient immune suppression would prevent the antivirus immune response. However, the long-term impact of even transient immunosuppression on the development of an immature immune system needs to be carefully evaluated.
In summary, we demonstrated that in utero injection of both Ad and AAV vectors between d 13 and 15 p.c. did not result in tolerance to the virus or transgene product in B6/129 F1 mice. It is understandable that induction of complete tolerance to a complex virus, such as an Ad, would be difficult. In this regard, our finding may help allay concerns that the application of fetal gene therapy using Ad vectors could lead to increased susceptibility to severe Ad infection in the future. On the other hand, tolerance to the therapeutic gene product is often desirable; and we suspect that this is still possible if the antigen is presented more effectively and at a critical time in fetal development.
Abbreviations
- Ad:
-
adenovirus
- AAV:
-
adeno-associated virus
- p.c.:
-
postconception
- i.m.:
-
intramuscular
- i.h.:
-
intrahepatic
- i.a.:
-
intra-amniotic
- Nab:
-
neutralizing antibody
- GC:
-
genome copies
- HSC:
-
hematopoietic stem cells
- Ad.CBlacZ:
-
recombinant adenovirus carrying Escherichia coli lacZ gene controlled by the cytomegalovirus enhancer and chicken β-actin gene promoter
- Ad.CMVhGH:
-
recombinant adenovirus carrying hGH controlled by cytomegalovirus promoter
- AAV.CMVlacZ:
-
recombinant AAV vector carrying E. coli lacZ gene controlled by the cytomegalovirus promoter
- AAV.CMVhGH:
-
recombinant AAV vector carrying hGH controlled by the cytomegalovirus promoter
- hGH:
-
human growth hormone
- lacZ:
-
E. coli lac Z gene encoding β-galactosidase
References
Turkay A, Saunders T, Kurachi K 1999 Intrauterine gene transfer: gestational stage-specific gene delivery in mice. Gene Ther 6: 1685–1694
Sekhon H, Larson J 1995 In utero gene transfer into the pulmonary epithelium. Nat Med 1: 1201–1203
Wang G, Williamson R, Mueller G, Thomas P, Davidson BL, McGray PB 1998 Ultrasound-guided gene transfer to hepatocytes in utero. Fetal Diagn Ther 13: 197–205
Vincent MC, Trapnell BC, Baughman RP, Wert SE, Whitsett JA, Iwamoto HS 1995 Adenovirus-mediated gene transfer to the respiratory tract of fetal sheep in utero. Hum Gene Ther 6: 1019–1020
Mitchell M, Jerebtsova M, Batshaw ML, Newman K, Ye X 2000 Long-term gene transfer to mouse fetuses with recombinant adenovirus adeno-associated virus (AAV) vectors. Gene Ther 7: 1986–1992
Lipshutz G, Gruber CA, Cao Y, Harby J, Contag CH, Gaensler KM 2001 In utero delivery of adeno-associated viral vectors: intraperitonial gene transfer produces long-term expression. Mol Ther 3: 284–292
Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, Wilson JM 2000 Humoral immunity to adeno-associated virus type 2 vector following administration to murine nonhuman primate muscle. J Virol 74: 2420–2425
Yang Y, Jooss KU, Su Q, Ertl HC, Wilson JM 1996 Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther 3: 137–144
Yang Y, Li Q, Ertl HC, Wilson JM 1995 Cellular humoral immune responses to viral antigens create barriers to lung-directed gene therapy with recombinant adenoviruses. J Virol 69: 2004–2015
Iwamoto H 1996 The window of opportunity for fetal gene therapy. Mol Hum Reprod 2: 472–474
Scott D 1994 The Nature of Immunologic Tolerance. Landes, Austin, TX, p 157
Brusilow S, Horwich A 1995 Urea cycle enzyme. In: Scriver C, Beaudet A, Sly W, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease, 7th ed. McGraw-Hill, New York, pp 1187–1232
Crombleholme T, Bianchi D 1994 In utero hematopoietic stem cell transplantation gene therapy. Semin Perinatol 18: 376–384
Flake A, Harrison M, Zanjani E 1991 In utero stem cell transplantation. Exp Hematol 19: 1061–1064
Iwamoto HS, Trapnell BC, McConnell CJ, Daugherty C, Whitsett JA 1999 Pulmonary inflammation associated with repeated, prenatal exposure to an E1, E3-deleted adenoviral vector in sheep. Gene Ther 6: 98–106
Yang EY, Kim HB, Shaaban AF, Milner R, Adzick NS, Flake AW 1999 Persistent postnatal transgene expression in both muscle liver after fetal injection of recombinant adenovirus. J Pediatr Surg 34: 766–773
Kozarsky K, Grossman M, Wilson JM 1993 Adenovirus-mediated correction of the genetic defect in hepatocytes from patients with familial hypercholesterolemia. Somat Cell Mol Genet 19: 449–458
Ye X, Robinson MB, Batshaw ML, Furth EE, Smith I, Wilson JM 1996 Prolonged metabolic correction in adult ornitine transcarbamilase-deficient mice with adenoviral vectors. J Biol Chem 271: 3639–3646
Davis A, Wilson J 1996 Adenoviral vectors. In: Dracopoli NC, Haines JL, Korf BR, Morton CC, Seidman CE, Seidman JG, Smith DR (eds) Current Protocols in Human Genetics. Wiley, New York, pp 12.4.7–12.4.15
Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM 1999 Gene therapy vectors based on adeno-associated virus type I. J Virol 73: 3994–4003
Lipshutz G, Flebbe-Rehwaldt L, Gfaensler KM 2000 Reexpression following readministration of an adenoviral vector in adult mice after initial in utero adenoviral administration. Mol Ther 2: 374–380
Billingham R, Brent L, Medawar P 1953 Actively acquired tolerance to foreign cells. Nature 172: 603–605
Carrier E, Lee T, Busch M, Cowan M 1995 Induction of tolerance in nondefective mice after in utero transplantation of major histocompatibility complex-mismatched fetal hematopoietic stem cells. Blood 86: 4681–4690
Fleischman R, Mintz B 1984 Development of adults bone marrow stem cells in H-2 compatible incompatible mouse fetuses. J Exp Med 159: 731–745
Fleischman R, Mintz B 1979 Prevention of genetic anemias in mice by microinjection of normal hematopoietic stem cells into the fetal placenta. Proc Natl Acad Sci USA 76: 5736–5740
Brockstedt D, Podsakoff GM, Fong L, Kurtzman G, Mueller-Ruchholtz W, Engleman EG 1999 Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin Immunol 92: 67–75
Xiao W, Chirmule N, Schnell MA, Tazelaar J, Huges JV, Wilson JM 2000 Route of administration determines induction of T-cell-independent humoral responses to adeno-associated virus vectors. Mol Ther 1: 323–329
Douagi I, Andre I, Ferraz JC, Cumano A 2000 Characterization of T cell precursor activity in the murine fetal thymus: evidence for an input of T cell precursors between days 12 14 gestation. Eur J Immunol 30: 2201–2210
Strasser A, Rolink A, Melchers F 1989 One synchronous wave of B cell development in mouse fetal liver changes at day 16 of gestation from dependence to independence of a stromal cell environment. J Exp Med 170: 1973–1986
Zinkernagel RM 2000 Localization dose time of antigens determine immune reactivity. Semin Immunol 12: 163–171
Fields PA, Armstrong E, Hagstrom JN, Arruda VR, Murphy ML, Farrell JP, High KA, Herzog RW 2001 Intravenous administration of an E1/E3-deleted adenoviral vector induces tolerance to factor IX in C57BL/6 mice. Gene Ther 8: 354–361
Juillard V, Villefroy P, Godfrin D, Pavirani A, Venet A, Guillet JG 1995 Long-term humoral cellular immunity induced by a signal immunization with replication-defective adenovirus recombinant vector. Eur J Immunol 25: 3467–3473
Michou AI, Santoro L, Christ M, Julliard V, Pavirani A, Mehtali MV 1997 Adenovirus-mediated gene transfer: influence of transgene, mouse strain type of immune response on persistence of transgene expression. Gene Ther 4: 473–482
DeMatteo RP, Chu G, Ahn M, Chang E, Barker CF, Markmann JF 1997 Long-lasting adenovirus transgene expression in mice through neonatal intrathymic tolerance induction without the use of immunosuppression. J Virol 71: 5330–5335
Camargo F, Huey-Louie D, Sassani A, Finn A, Schneider D, Agah R, Dichek D 2000 Adenovirus-transgenic mice: partial embrionic lethality lack of immunological tolerance to adenoviral vectors. Mol Ther 1: S180–S181( abstr)
Ridge JP, Fuchs EJ, Matzinger P 1996 Neonatal tolerance revisited: turning on newborn T cells with dendritic cells. Science 271: 1726–1728
Gallucci S, Matzinger P 2001 Danger signals: SOS to the immune system. Curr Opin Immunol 13: 114–119
Tran ND, Porada CD, Almeida-Porada G, Glimp, Anderson WF, Zanjani ED 2001 Induction of stable prenatal tolerance to b-galactosidase by in utero gene transfer into preimmune sheep fetuses. Blood 97: 3417–3423
Hackett NR, Kaminsky SM, Sondhi D, Crystal RG 2000 Antivector antitransgene host responses in gene therapy. Curr Opin Mol Ther 2: 376–382
Kearns WG, Afione SA, Fulmer SB, Pang MC, Erikson D, Egan M, Landrum MJ, Flotte TR, Cutting GR 1996 Recombinant adeno-associated virus (AAV-CFTR) vectors do not integrate in a site-specific fashion in an immortalized epithelial cell line. Gene Ther 3: 748–755
Fisher KD, Stallwood Y, Green NK, Ulbrich K, Mautner V, Seymour LW 2001 Polymer-coated adenovirus permits efficient retargeting evades neutralizing antibodies. Gene Ther 8: 341–348
Reynolds PN, Dimitriev I, Curiel DT 1999 Insertion of an RGD motif into the H1 loop of adenovirus fiber protein alters the distribution of transgene expression of the systemically administered vector. Gene Ther 6: 1336–1339
Gu DL, Gonzalez AM, Printz MA, Doukas J, Ying W, D'Andrea M, Hoganson DK, Curiel DT, Douglas JT, Sosnowski BA, Baird A, Aukerman SL, Pierce GF 1999 Fibroblast growth factor 2 retargeted adenovirus has redirected cellular tropism: evidence for reduced toxicity enhanced antitumor activity in mice. Cancer Res 59: 2608–2614
Morral N, O'Neal W, Rice K, Leland M, Kaplan J, Piedra PA, Zhou H, Parks RJ, Velji R, Aguilar-Cordova E, Wadsworth S, Graham FL, Kochanek S, Carey KD, Beaudet AL 1999 Administration of helper-dependent adenoviral vectors sequential delivery of different vector serotype for long-term liver-directed gene transfer in baboons. Proc Natl Acad Sci USA 96: 12816–12821
Parks R, Evelegh C, Graham F 1999 Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther 6: 1565–1573
Jooss K, Yang Y, Wilson JM 1996 Cyclophosphamide diminishes inflammation prolong transgene expression following delivery of adenoviral vectors to mouse liver lung. Hum Gene Ther 7: 1555–1566
Filds Pa, Arruda R, Armstrong E, Chu K, Mingozzi F, Hagston JN, Herzog RW, High KA 2001 Risk preventation of anti-factor IX formation in AAV-mediated gene transfer in the context of a large deletion of F9. Mol Ther 4: 201–210
Ye X, Robinson MB, Pabin C, Batshaw ML, Wilson JM 2000 Transient depletion of CD4 lymphocyte improves efficacy of repeted administration of recombinant adenovirus in the ornithine transcarbamylase deficient sparse fur mice. Gene Ther 7: 1761–176
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported by National Institutes of Health grant P30HD 40677, and a grant from the Kettering Family Foundation.
Rights and permissions
About this article
Cite this article
Jerebtsova, M., Batshaw, M. & Ye, X. Humoral Immune Response to Recombinant Adenovirus and Adeno-Associated Virus after In Utero Administration of Viral Vectors in Mice. Pediatr Res 52, 95–104 (2002). https://doi.org/10.1203/00006450-200207000-00018
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200207000-00018
This article is cited by
-
Effect of viral dose on neutralizing antibody response and transgene expression after AAV1 vector re-administration in mice
Gene Therapy (2008)
-
Persistent Expression of hF.IX After Tolerance Induction by In Utero or Neonatal Administration of AAV-1-F.IX in Hemophilia B Mice
Molecular Therapy (2007)
-
Immune response to lentiviral bilirubin UDP-glucuronosyltransferase gene transfer in fetal and neonatal rats
Gene Therapy (2006)
-
Comparison of high-capacity and first-generation adenoviral vector gene delivery to murine muscle in utero
Gene Therapy (2005)
-
Immune responses to adeno-associated virus and its recombinant vectors
Gene Therapy (2003)