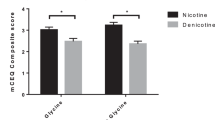
Abstract
Stress and hormones released in response to stress influence the effects of nicotine and the severity of nicotine withdrawal. Here, we systematically examine the contribution of a stress response gene, FKBP5, to the acute and chronic behavioral effects of nicotine in smokers. Subjects were European- and African-American (EA and AA) heavy smokers who participated in an intravenous (IV) nicotine administration study (total n=169). FKBP5 rs3800373 genotype was analyzed for association to several outcomes, including nicotine withdrawal and the acute subjective, heart rate (HR), blood pressure and plasma cortisol responses to IV nicotine. Nicotine withdrawal was also examined in relation to rs3800373 allele frequencies in an independent cohort of EA and AA current smokers (n=3821). For a subset of laboratory subjects FKBP5 mRNA (n=48) expression was explored for an association to the same outcomes. The rs3800373 minor allele was associated with less severe nicotine withdrawal in laboratory subjects and the independent cohort of smokers. The rs3800373 minor allele was also associated with lower subjective ratings of negative drug effects in response to IV nicotine. Low FKBP5 mRNA expression was associated lower cortisol levels, lower subjective ratings of negative drug effects and a blunted HR response to nicotine. Stress hormone regulation via FKBP5 warrants further investigation as a potential contributor to the effects of nicotine withdrawal, which occurs commonly, and has an important role in the maintenance of smoking behavior and relapse following a quit attempt.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sinha R . How does stress increase risk of drug abuse and relapse? Psychopharmacology 2001; 158: 343–359.
Hapke U, Schumann A, Rumpf HJ, John U, Konerding U, Meyer C . Association of smoking and nicotine dependence with trauma and posttraumatic stress disorder in a general population sample. J Nerv Ment Dis 2005; 193: 843–846.
Beckham JC, Calhoun PS, Dennis MF, Wilson SM, Dedert EA . Predictors of lapse in first week of smoking abstinence in PTSD and non-PTSD smokers. Nicotine Tob Res 2013; 15: 1122–1129.
Breslau N, Davis GC, Schultz LR . Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch Gen Psychiatry 2003; 60: 289–294.
Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ . Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry 1996; 40: 79–88.
Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000; 47: 769–776.
Menke A, Arloth J, Putz B, Weber P, Klengel T, Mehta D et al. Dexamethasone stimulated gene expression in peripheral blood is a sensitive marker for glucocorticoid receptor resistance in depressed patients. Neuropsychopharmacology 2012; 37: 1455–1464.
Menke A, Klengel T, Rubel J, Bruckl T, Pfister H, Lucae S et al. Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behav 2013; 12: 289–296.
Yehuda R, Cai G, Golier JA, Sarapas C, Galea S, Ising M et al. Gene expression patterns associated with posttraumatic stress disorder following exposure to the World Trade Center attacks. Biol Psychiatry 2009; 66: 708–711.
Klengel T, Mehta D, Anacker C, Rex-Haffner M, Pruessner JC, Pariante CM et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci 2013; 16: 33–41.
Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB et al. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA 2008; 299: 1291–1305.
Binder EB, Salyakina D, Lichtner P, Wochnik GM, Ising M, Putz B et al. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat Genet 2004; 36: 1319–1325.
Xie P, Kranzler HR, Poling J, Stein MB, Anton RF, Farrer LA et al. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology 2010; 35: 1684–1692.
Roy A, Gorodetsky E, Yuan Q, Goldman D, Enoch MA . Interaction of FKBP5, a stress-related gene, with childhood trauma increases the risk for attempting suicide. Neuropsychopharmacology 2010; 35: 1674–1683.
Huang MC, Schwandt ML, Chester JA, Kirchhoff AM, Kao CF, Liang T et al. FKBP5 moderates alcohol withdrawal severity: human genetic association and functional validation in knockout mice. Neuropsychopharmacology 2014; 39: 2029–2038.
Hubler TR, Scammell JG . Intronic hormone response elements mediate regulation of FKBP5 by progestins and glucocorticoids. Cell Stress Chaperones 2004; 9: 243–252.
Westberry JM, Sadosky PW, Hubler TR, Gross KL, Scammell JG . Glucocorticoid resistance in squirrel monkeys results from a combination of a transcriptionally incompetent glucocorticoid receptor and overexpression of the glucocorticoid receptor co-chaperone FKBP51. J Steroid Biochem Mol Biol 2006; 100: 34–41.
Matta SG, Beyer HS, McAllen KM, Sharp BM . Nicotine elevates rat plasma ACTH by a central mechanism. J Pharmacol Exp Ther 1987; 243: 217–226.
Mendelson JH, Sholar MB, Goletiani N, Siegel AJ, Mello NK . Effects of low- and high-nicotine cigarette smoking on mood states and the HPA axis in men. Neuropsychopharmacology 2005; 30: 1751–1763.
Shi LJ, He HY, Liu LA, Wang CA . Rapid nongenomic effect of corticosterone on neuronal nicotinic acetylcholine receptor in PC12 cells. Arch Biochem Biophys 2001; 394: 145–150.
Ke L, Lukas RJ . Effects of steroid exposure on ligand binding and functional activities of diverse nicotinic acetylcholine receptor subtypes. J Neurochem 1996; 67: 1100–1112.
Caggiula AR, Donny EC, Epstein LH, Sved AF, Knopf S, Rose C et al. The role of corticosteroids in nicotine's physiological and behavioral effects. Psychoneuroendocrinology 1998; 23: 143–159.
Bruijnzeel AW, Prado M, Isaac S . Corticotropin-releasing factor-1 receptor activation mediates nicotine withdrawal-induced deficit in brain reward function and stress-induced relapse. Biol Psychiatry 2009; 66: 110–117.
al'Absi M, Hatsukami D, Davis GL, Wittmers LE . Prospective examination of effects of smoking abstinence on cortisol and withdrawal symptoms as predictors of early smoking relapse. Drug Alcohol Depend 2004; 73: 267–278.
Frederick SL, Reus VI, Ginsberg D, Hall SM, Munoz RF, Ellman G . Cortisol and response to dexamethasone as predictors of withdrawal distress and abstinence success in smokers. Biol Psychiatry 1998; 43: 525–530.
Herman AI, Jatlow PI, Gelernter J, Listman JB, Sofuoglu M . COMT Val158Met modulates subjective responses to intravenous nicotine and cognitive performance in abstinent smokers. Pharmacogenomics J 2013; 13: 490–497.
Sofuoglu M, Herman AI, Nadim H, Jatlow P . Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology 2012; 37: 1509–1516.
First MB, Spitzer RL, Gibbon M, Williams JBW . The structured clinical interview for Dsm-Iii-R personality-disorders (Scid-Ii).1. description. J Pers Disord 1995; 9: 83–91.
Hughes JR, Hatsukami D . Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 1986; 43: 289–294.
Watson D, Clark LA, Tellegen A . Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54: 1063–1070.
Tiffany ST, Drobes DJ . The development and initial validation of a questionnaire on smoking urges. Br J Addict 1991; 86: 1467–1476.
Gelernter J, Kranzler HR, Sherva R, Almasy L, Koesterer R, Smith AH et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol Psychiatry 2013; 19: 41–49.
Gelernter J, Sherva R, Koesterer R, Almasy L, Zhao H, Kranzler HR et al. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol Psychiatry 2013; 19: 717–723.
Gelernter J, Kranzler HR, Sherva R, Koesterer R, Almasy L, Zhao H et al. Genome-wide association study of opioid dependence: multiple associations mapped to calcium and potassium pathways. Biol Psychiatry 2013; 76: 66–74.
Gelernter J, Kranzler HR, Sherva R, Almasy L, Herman AI, Koesterer R et al. Genomewide association study of nicotine dependence in american populations: identification of novel risk loci in both african- and European-Americans. Biol Psychiatry 2014.
Pierucci-Lagha A, Gelernter J, Feinn R, Cubells JF, Pearson D, Pollastri A et al. Diagnostic reliability of the Semi-structured assessment for drug dependence and alcoholism (SSADDA). Drug Alcohol Depend 2005; 80: 303–312.
Bevilacqua L, Carli V, Sarchiapone M, George DK, Goldman D, Roy A et al. Interaction between FKBP5 and childhood trauma and risk of aggressive behavior. Arch Gen Psychiatry 2012; 69: 62–70.
Yang BZ, Zhao H, Kranzler HR, Gelernter J . Practical population group assignment with selected informative markers: characteristics and properties of Bayesian clustering via STRUCTURE. Genet Epidemiol 2005; 28: 302–312.
Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O'Malley SS . The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology 2013; 227: 177–192.
Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81: 559–575.
West RJ, Hajek P, Belcher M . Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med 1989; 19: 981–985.
Heilig M, Egli M, Crabbe JC, Becker HC . Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol 2010; 15: 169–184.
Koob G, Kreek MJ . Stress, dysregulation of drug reward pathways, and the transition to drug dependence. A J Psychiatry 2007; 164: 1149–1159.
Piazza PV, Rouge-Pont F, Deroche V, Maccari S, Simon H, Le Moal M . Glucocorticoids have state-dependent stimulant effects on the mesencephalic dopaminergic transmission. Proc Natl Acad Sci USA 1996; 93: 8716–8720.
Graf EN, Wheeler RA, Baker DA, Ebben AL, Hill JE, McReynolds JR et al. Corticosterone acts in the nucleus accumbens to enhance dopamine signaling and potentiate reinstatement of cocaine seeking. J Neurosci 2013; 33: 11800–11810.
Marcinkiewcz CA, Prado MM, Isaac SK, Marshall A, Rylkova D, Bruijnzeel AW . Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology 2009; 34: 1743–1752.
Pauly JR, Ullman EA, Collins AC . Adrenocortical hormone regulation of nicotine sensitivity in mice. Physiol Behav 1988; 44: 109–116.
Robinson SF, Grun EU, Pauly JR, Collins AC . Changes in sensitivity to nicotine and brain nicotinic receptors following chronic nicotine and corticosterone treatments in mice. Pharmacol Biochem Behav 1996; 54: 587–593.
Pauly JR, Grun EU, Collins AC . Chronic corticosterone administration modulates nicotine sensitivity and brain nicotinic receptor binding in C3H mice. Psychopharmacology 1990; 101: 310–316.
Scammell JG, Denny WB, Valentine DL, Smith DF . Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol 2001; 124: 152–165.
Cohen LM, al'Absi M, Collins FL Jr . Salivary cortisol concentrations are associated with acute nicotine withdrawal. Addict Behav 2004; 29: 1673–1678.
Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J et al. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry 2005; 62: 1258–1265.
Reuter M, Netter P, Rogausch A, Sander P, Kaltschmidt M, Dorr A et al. The role of cortisol suppression on craving for and satisfaction from nicotine in high and low impulsive subjects. Hum Psychopharmacol 2002; 17: 213–224.
Barrett JC, Fry B, Maller J, Daly MJ . Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 2005; 21: 263–265.
Acknowledgements
This work was supported in part by NIH grants, R01 AA011330, R01 AA017535, R01 DA030976, R01 DA12690, R03 DA027474, K24 AA013736, K12 DA00167, T32 MH014276 and T32 AA015496. This work also received support from the US Department of Veterans Affairs (VA) through Cooperative Study #575B, the VISN 1 New England Mental Illness Research, Education and Clinical Center (MIRECC) and VA National Center for PTSD, and a VA VISN 1 CDA and VA MERIT. Genotyping services were provided by the Center for Inherited Disease Research (CIDR). CIDR is fully funded through a federal contract from the National Institutes of Health to The Johns Hopkins University (contract number N01-HG-65403).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Unrelated to this research, Dr Kranzler has been a consultant or advisory board member for Alkermes, Lilly, Lundbeck, Otsuka, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which is supported by AbbVie, Ethypharm, Lilly, Lundbeck and Pfizer and has a US patent pending entitled, ‘Test for Predicting Response to Topiramate and Use of Topiramate’. Dr Sofuoglu has served as an expert witness on behalf of Pfizer in lawsuits related to varenicline. All other authors declare no potential conflict of interest.
Additional information
Supplementary Information accompanies the paper on the The Pharmacogenomics Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Jensen, K., Herman, A., Morean, M. et al. FKBP5 variation is associated with the acute and chronic effects of nicotine. Pharmacogenomics J 15, 340–346 (2015). https://doi.org/10.1038/tpj.2014.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2014.76
This article is cited by
-
Threshold dose for intravenous nicotine self-administration in young adult non-dependent smokers
Psychopharmacology (2021)
-
Differential effects of nicotine delivery rate on subjective drug effects, urges to smoke, heart rate and blood pressure in tobacco smokers
Psychopharmacology (2020)
-
Intravenous Nicotine Self-Administration in Smokers: Dose–Response Function and Sex Differences
Neuropsychopharmacology (2016)
-
Gene–Stress–Epigenetic Regulation of FKBP5: Clinical and Translational Implications
Neuropsychopharmacology (2016)
-
A CHRNA5 Smoking Risk Variant Decreases the Aversive Effects of Nicotine in Humans
Neuropsychopharmacology (2015)