Abstract
The inability of vaccines to retain sufficient thermostability has been an obstacle to global vaccination programs. To address this major limitation, we utilized carbohydrate-based ice recrystallization inhibitors (IRIs) to eliminate the cold chain and stabilize the potency of Vaccinia virus (VV), Vesicular Stomatitis virus (VSV) and Herpes virus-1 (HSV-1). The impact of these IRIs was tested on the potency of the viral vectors using a plaque forming unit assay following room temperature storage, cryopreservation with successive freeze-thaw cycles and lyophilization. Viral potency after storage with all three conditions demonstrated that N-octyl-gluconamide (NOGlc) recovered the infectivity of shelf stored VV, 5.6 Log10 PFU mL−1 during 40 days and HSV-1, 2.7 Log10 PFU mL−1 during 9 days. Carbon-linked antifreeze glycoprotein analogue ornithine-glycine-glycine-galactose (OGG-Gal) increases the recovery of VV and VSV more than 1 Log10 PFU mL−1 after 10 freeze-thaw cycles. In VSV, cryostorage with OGG-Gal maintains high infectivity and reduces temperature-induced aggregation of viral particles by 2 times that of the control. In total, OGG-Gal and NOGlc preserve virus potency during cryostorage. Remarkably, NOGlc has potential to eliminate the cold chain and permit room temperature storage of viral vectors.
Similar content being viewed by others
Vaccination is a public health success story saving 3 million lives each year. Many vaccines contain live attenuated viruses that have been cultivated under conditions that disable their virulent properties, or closely related but less dangerous viral vectors to produce a broad immune response or/and an anti-cancer (oncolytic) effect. The inability of viral vaccines to retain sufficient thermostability has been a significant obstacle to global vaccination programs and viral-based therapy1,2. Elevated temperatures damage live viruses and while cryopreservation is the preferred method of storage, freezing dramatically reduces the titer of the virus. Furthermore, exposure to low temperatures associated with cryopreservation results in virus agglomeration, rendering the vaccine ineffective3,4. The World Health Organization has mandated that, in order for a vaccine to be considered “effective”, less than 1 Log10 decrease in the original titer is tolerated5,6. Several approaches like biomineralization, addition of silk or albumin have been applied but unfortunately they often have multi-step preparation protocols or cause unwanted immune responses3,4,5,7,8,9,10,11,12,13. Consequently, novel methods for the preservation of vaccines are urgently required.
In this work, we examined the activity and thermostability of three live viral vectors from vesicular stomatitis virus (VSV), vaccinia virus (VV) and herpes simplex virus type 1 (HSV-1), all of which are popular candidates for cancer vaccine development. For example, VSV-Δ51, has been shown to possess potent oncolytic properties14 against a large number of potential tumor types15. VSV is a small bullet-shaped negative-strand RNA virus from the Rhabdoviridae family16. VSV selectively attacks tumor cells by taking advantage of defects to the interferon pathway17. Subsequently, VSV has been considered for clinical trials by Recombinant DNA Advisory Committee of NIH18,19. Furthermore, VSV is being developed as a vaccine shuttle for an array of viral pathogens, such as HIV-120, Ebola virus21, hepatitis B22 and C23. JX-594 strain of VV is a member of the poxvirus family and has a large linear double-stranded DNA genome of approximately 200 kbp in length that encodes ~ 250 genes. It has several attributes that make it particularly well suited as an anticancer therapeutic24. VV is designed to attack cancer through three diverse mechanisms of action: 1) the lysis of cancer cells through viral replication, 2) the reduction of the blood supply to tumors through vascular targeting and destruction and 3) the stimulation of the body's immune response against cancer cells. An attenuated HSV-1 strain, known as talimogene laherparepvec is a DNA oncolytic virus currently being studied for the treatment of melanoma and other advanced cancers by Amgen. With the announcement of positive results in March 2013, it is the first oncolytic virus to be proven effective in a Phase III clinical trial.
We examined thirteen compounds (all compounds can be found in Supporting Information) to preserve VV, HSV-1 and VSV at room temperature storage, during successive freeze-thaw cycles and lyophilization and identified three promising ice recrystallization inhibitors, ornithine-glycine-glycine-galactose (OGG-Gal), N-octyl-d-gluconamide (NOGlc) and N-octyl-d-galactonamide (NOGal) (Figure 1a). All of these compounds demonstrate the ability to inhibit ice recrystallization3,25 and significantly increase the infectivity and thermostability of the viruses. OGG-Gal is a C-linked analogue of naturally occurring antifreeze glycoproteins (AFGPs)26 which allow arctic fish to survive in sub-zero temperatures27. OGG-Gal has been shown to be a potent inhibitor of ice recrystallization without the property of thermal hysteresis26, which can be detrimental to biological samples at low temperatures. Until recently, many structure-function studies of native AFGPs were only assessed for thermal hysteresis activity and not ice recrystallization activity28. However, more recent studies have explored the relationships between AFGP analogues and IRI activity29. The open-chain carbohydrates or alditols, NOGlc and NOGal, are hydrogelators that are capable of immobilizing water molecules into three-dimensional networks having morphologies of fibers30,31. Their ability to inhibit ice recrystallization was assessed using the standard “splat-cooling” assay26,32 in which ice crystal size was determined using photographs of frozen ice wafers after a 30 min annealing time at -6.4°C. The mean grain ice crystal size was determined using domain recognition software33 and compared to a phosphate buffered saline (PBS) solution as a control. As illustrated in Figure 1b, OGG-Gal is an extremely potent IRI at a concentration of 5.5 μM. In comparison, alditol NOGlc exhibits potent IRI activity only at 500 μM. Shortening the hydrophobic side chain in NOGlc results in a loss of IRI activity, indicating that the amphiphilic nature of these alditols is an essential property34. Interestingly, replacing the glucose alditol portion of NOGlc with a galactose alditol portion (NOGal) results in weak to moderate IRI activity, indicating that the stereochemical arrangement of the hydroxyl groups in the polyol component is an essential structural feature necessary for potent IRI activity as these two compounds only differ by one single stereocentre (indicated by boxes in Figure 1a). It is hypothesized that the mechanism by which these compounds inhibit ice recrystallization is though the disruption of the bulk water present between ice crystal boundaries35. As ice crystals grow, all solutes are excluded and the ice recrystallization inhibitors (IRIs) are concentrated at the interface of two ice crystals, where the interface consists of semi-ordered ice (quasi-liquid layer) separated by a layer of bulk water. It is thought that the hydration shell of the IRIs disrupts the ordering of bulk water therefore causing an increase in energy for the transfer of water molecules from bulk water to the ice lattice. Although the structure and molecular weights of OGG-Gal and the small IRIs is significantly different, the mechanism for ice recrystallization is thought to be the same. Further studies must be performed to determine the toxicity and immunogenicity of these compounds in animals.
The most potent compound resulting in stabilization of VV and HSV-1 at room temperature is NOGlc. VV and HSV-1 with and without 250 μM NOGlc were stored at 22°C and the infectivity of the viruses was determined counting plaque-forming units (PFU) in a cell-based assay. These data are represented by curves of logarithmic infectivity versus time (Figure 2). As a control, the infectivity of VV in PBS declined more than 1 Log10 PFU mL−1 in the first 8 days of storage and the ability to infect host cells was completely lost after 40 days. However, in the presence of NOGlc the infectivity decreased by only 0.2 Log10 PFU mL−1 after 40 days storage at room temperature (Figure 2a). These results were confirmed by viral quantitative capillary electrophoresis (viral qCE)36, where the intact virus particles were separated from free DNA present after virus degradation (Figure S1). VV degraded slower when incubated with NOGlc than with PBS as more VV peaks and less DNA was observed. We also tested the ability of NOGlc to stabilize HSV-1 at ambient temperature. While the reduction of untreated HSV-1 infectivity was 1 Log10 PFU mL−1 after one day, NOGlc treated HSV-1 lost only 0.05 Log10 PFU mL−1 of its infectivity on the first day and only 0.41 Log10 PFU mL−1 after 9 days, the maximum number of tested days for HSV-1 (Figure 2b).
While working with VV and VSV we found that even after multiple exposures to −20°C temperatures, the addition of carbohydrate-based IRIs prevented the damage caused by successive freezing and thawing. For instance, VV treated with OGG-Gal, NOGal and NOGlc lost 0.3, 1.3 and 0.5 Log10 PFU mL−1 respectively after 10 freeze-thaw cycles, compared with 1.7 Log10 PFU mL−1 lost in a control experiment with PBS (Figure 3a). Titration experiments demonstrate that the protective effect and ultimately the infectivity of VV was improved with increasing concentrations of NOGlc. The maximum protective effect with NOGal for VV was observed at 62.5 nM (Figure S2a and Figure S2b). The infectivity of VSV in the presence of OGG-Gal and NOGlc was almost 2.1 Log10 higher than a control after 10 freeze-thaw cycles (Figure 3b). Titration experiments revealed the maximum protection of VSV at 31 μM of NOGlc and 3 μM of OGG-Gal Figure S2d and S2c).
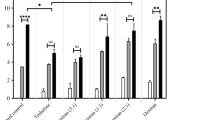
Regardless of how effective a vaccine may be in the laboratory, its commercial potential will be limited due to poor stability during storage and distribution. Lyophilization (freeze-drying) is a well-established technique used in the pharmaceutical industry for stabilizing high-cost, labile bio-products, such as vaccines. We examined the ability of our carbohydrate-based IRIs to preserve VV and VSV during the lyophilization process. As controls, we had tested 2% bovine serum albumin, fetal calf serum and glycerol as preservers just prior the test virus lyophilization. The recovery of the test viruses was the same as diluted viruses in PBS. Prior to lyophilization, VV infectivity was 6 Log10 PFU mL−1 but in the presence of OGG-Gal, NOGal, NOGlc and PBS VV infectivity was reduced by 0.68, 1.37, 1.06 and 2 Log10 PFU mL−1, respectively (Figure 4a). OGG-Gal showed the most promising result with only 0.68 Log10 PFU mL−1 reduction compared to 2 Log10 of PBS control. The infectivity of VSV in the presence of OGG-Gal, NOGal, NOGlc and PBS was reduced by 5–6 Log10 PFU mL−1 (Figure 4b). The question of why do these compounds protect viral vectors appears to be complex, as the same compounds provide protection with both storage methods. It is accepted that the majority of damage to bio-materials during cryopreservation is due to ice recrystallization that occurs during the storage and thawing cycles (assuming adequate dehydration during the rate controlled freezing process as described by Mazur's two stage hypothesis of cryoinjury). In lyophilization, samples are flash frozen and ice crystals are removed by sublimation. Under both conditions it seems reasonable that the ability of an ice recrystallization inhibitor to mitigate ice growth would be beneficial and ultimately protect against cryoinjury in both processes. However, another reason for decreased infectivity in cryopreserved and lyophilized samples is aggregation or agglomeration of virus particles. Consequently, we evaluated the impact of OGG-Gal on VSV aggregation after one freeze-thaw cycle using two different VSV expressing either yellow fluorescent protein (YPF) or red fluorescence protein (RFP). Galasso G. et al. by using electron microscopy showed that when a cell monolayer is infected with a low proportion of virus, one active viral particle can infect one cell and an aggregation several infectious particles lead only one cell infection37. An overlay of 1% Agarose restricts the spread of virus to neighbor cells. Hence, in this assay, a single virus with either RFP or YFP will infect a single host cell. In instances where aggregation of the virus particles occurs, multiple virus particles enter the cell and both red and yellow fluorescence is observed. After viral infection of cells with a mixture of two viruses, we detected fluorescent proteins and their localizations inside the cells (Figure 5). In the presence of OGG-Gal, the agglomeration of VSV dropped almost 2 times from 34 ± 3.8% to 16 ± 3.0% (Figure 5b). It is interesting to note that direct addition of the carbohydrate-based IRIs to a virus sample increases its infectivity by approximately 20% (Figure S3). These results suggest the compounds disperse virus aggregates and stabilize individual virus particles in the solution. The abilities of OGG-Gal, NOGal and NOGlc to stabilize and preserve three different viruses during cryopreservation, lyophilization and room temperature storage are summarized in Table 1.
Considering the structural differences between OGG-Gal, a high-molecular weight glycopeptide and NOGlc, a small-molecule non-ionic surfactant, it is interesting to see very little difference in infectivity. Both compounds are equally effective at protecting against cryo-injury after ten successive freeze-thaw cycles. Furthermore, both of these compounds are most effective with VSV but less effective in VV. In contrast, NOGal is not effective (infectivity is identical to the PBS control). The IRI activity of small molecules is presented in Figure 1b. Ice recrystallization inhibition is defined as the prevention of the re-organization of smaller ice crystals into larger ice crystals. This would occur during the thawing phase in the freeze-thaw cycles. Both OGG-Gal and NOGlc are potent inhibitors of ice recrystallization. NOGlc is effective at 0.5 mM. Given the effectiveness of this compound to inhibit ice recrystallization, which is a significant cause of cellular damage during cryopreservation, it seems likely that NOGlc (and OGG-Gal) may directly inhibiting cryoinjury of the virus particle. In contrast, NOGal does not inhibit ice recrystallization (despite the fact that it differs from NOGlc by only one stereocentre) to any appreciable extent and fails to protect VV and VSV against the cryoinjury associated with successive freezing-thawing. This result is also consistent with the hypothesis that inhibiting ice recrystallization injury in the virus particle is beneficial. Ice crystals can damage a large virus more than a small virus. For cryopreservation, the best compounds are amphiliphilc molecules possessing a hydrophilic head group and a hydrophobic tail group, as seen in the alditol structure NOGlc.
Maintaining the stability of viral vectors is an obstacle to global vaccination programs and viral-based therapy1,2,12,38,39. Unfortunately, most vaccines lose 50% of their activity when constructed and stored for one hour at room temperature6,9,38. The transportation, storage and use of them consequently present challenges, particularly in developing countries. Unlike former efforts, direct addition of NOGlc is easy, economical and robust. Our results show that NOGlc protects significantly VV and HSV-1 infectivity at room temperature for days (Figure 2).
In conclusion, according WHO requirement for vaccines cold chain management, NOGlc increases the shelf life of VV from four days to more than forty days and HSV-1 from a day to more than nine days. NOGlc has opportunity to eliminate the cold chain from viral vector vaccine management.
References
Brandau, D. T., Jones, L. S., Wiethoff, C. M., Rexroad, J. & Middaugh, C. R. Thermal stability of vaccines. J. Pharm. Sci. 92, 218–231 (2003).
Rexroad, J., Wiethoff, C. M., Jones, L. S. & Middaugh, C. R. Lyophilization and the thermostability of vaccines. Cell Preserv. Technol. 1, 91–104 (2002).
Kristensen, D., Chen, D. & Cummings, R. Vaccine stabilization: research, commercialization and potential impact. Vaccine 29, 7122–7124 (2011).
Matthias, D. M., Robertson, J., Garrison, M. M., Newland, S. & Nelson, C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine 25, 3980–3986 (2007).
Galazka, A., Milstien, J. & Zaffran, M. Thermostability of vaccines: global programme for vaccines and immunization. World Health Organization. Geneva (1998).
Knezevic, I. Stability evaluation of vaccines: WHO approach. Biologicals 37, 357–359 (2009).
Vrdoljak, A. et al. Coated microneedle arrays for transcutaneous delivery of live virus vaccines. J. Controlled Release 159, 34–42 (2012).
Milstien, J. B., Galazka, A. M., Kartoglu, U. M. & Zaffran, M. Temperature sensitivity of vaccines. WHO Document Production Services, pp. 1–73. (2006).
Greenwood, B., Salisbury, D. & Hill, A. V. Vaccines and global health. Philos. T. R. Soc. B. 366, 2733–2742 (2011).
Dietz, V., Galazka, A., Van Loon, F. & Cochi, S. Factors affecting the immunogenicity and potency of tetanus toxoid: implications for the elimination of neonatal and non-neonatal tetanus as public health problems. Bull. World Health Organ. 75, 81 (1997).
Parato, K. A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 20, 749–758 (2011).
Wang, G. et al. Eggshell-inspired biomineralization generates vaccines that do not require refrigeration. Angew. Chem. 124, 10728–10731 (2012).
Wang, G. et al. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc. Natl. Acad. Sci. U. S. A. 110, 7619–7624 (2013).
Cary, Z. D., Willingham, M. C. & Lyles, D. S. Oncolytic vesicular stomatitis virus Induces apoptosis in U87 glioblastoma cells by a type II death receptor mechanism and induces cell death and tumor clearance in vivo. J. Virol. 85, 5708–5717 (2011).
Barber, G. N. VSV-tumor selective replication and protein translation. Oncogene 24, 7710–7719 (2005).
Lyles, D. S. & Rupprecht, C. E. in Fields Virology Vol. 1 (eds Knipe M., & Howley M.) Ch. 39, 1364–1408 (Lippincott Williams & Wilkins, 2007).
Balachandran, S. & Barber, G. N. Vesicular stomatitis virus (VSV) therapy of tumors. IUBMB Life 50, 135–138 (2000).
Committee, R. D. A. Vol. December 3–4 (ed U.S. Department of Health and Human Services. ) (National Institutes of Health, Bethesda, 2008).
Committee, R. D. A. Vol. September 9–10 (ed U.S. Department of Health and Human Services. ) (National Institutes of Health, Bethesda, 2008).
Clarke, D. et al. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. Springer Semin. Immunopathol. 28, 239–253 (2006).
Geisbert, T. W. et al. Vesicular stomatitis virus-based ebola vaccine is well-tolerated and protects immunocompromised nonhuman primates. PLoS Path. 4, e1000225 (2008).
Cobleigh, M. A., Buonocore, L., Uprichard, S. L., Rose, J. K. & Robek, M. D. A vesicular stomatitis virus-based hepatitis B virus vaccine vector provides protection against challenge in a single dose. J. Virol. 84, 7513–7522 (2010).
Majid, A. M., Ezelle, H., Shah, S. & Barber, G. N. Evaluating replication-defective vesicular stomatitis virus as a vaccine vehicle. J. Virol. 80, 6993–7008 (2006).
Jacobs, B. L. et al. Vaccinia virus vaccines: past, present and future. Antiviral Res. 84, 1–13 (2009).
Williams, K. A. Synthesis and Characterization of Monosaccharide-derived Low Molecular Weight Gelators PhD thesis, University of New Orleans, (2011).
Czechura, P., Tam, R. Y., Dimitrijevic, E., Murphy, A. V. & Ben, R. N. The importance of hydration for inhibiting ice recrystallization with C-linked antifreeze glycoproteins. Journal of the American Chemical Society 130, 2928–2929 (2008).
Harding, M. M., Anderberg, P. I. & Haymet, A. D. J. ‘Antifreeze’ glycoproteins from polar fish. Eur. J. Biochem. 270, 1381–1392 (2003).
Tachibana, Y. et al. Antifreeze glycoproteins: elucidation of the structural motifs that are essential for antifreeze activity. Angew. Chem. Int. Ed. 43, 856–862 (2004).
Wilkinson, B. L. et al. Total synthesis of homogeneous antifreeze glycopeptides and glycoproteins. Angew. Chem. Int. Ed. 51, 3606–3610 (2012).
Pfannemüller, B. & Welte, W. Amphiphilic properties of synthetic glycolipids based on amide linkages. I. Electron microscopic studies on aqueous gels. Chem. Phys. Lipids 37, 227–240 (1985).
Fuhrhop, J. H., Schnieder, P., Boekema, E. & Helfrich, W. Lipid bilayer fibers from diastereomeric and enantiomeric N-octylaldonamides. J. Am. Chem. Soc. 110, 2861–2867 (1988).
Knight, C. A., Hallett, J. & DeVries, A. L. Solute effects on ice recrystallization: An assessment technique. Cryobiology 25, 55–60 (1988).
Jackman, J. et al. Assessing antifreeze activity of AFGP 8 using domain recognition software. Biochem. Biophys. Res. Commun. 354, 340–344 (2007).
Capicciotti, C. J. et al. Potent inhibition of ice recrystallization by low molecular weight carbohydrate-based surfactants and hydrogelators. Chem. Sci. 3, 1408–1416 (2012).
Tam, R. Y., Ferreira, S. S., Czechura, P., Chaytor, J. L. & Ben, R. N. Hydration index-A better parameter for explaining small molecule hydration in inhibition of ice recrystallization. J. Am. Chem. Soc. 130, 17494–17501 (2008).
Azizi, A. et al. Viral quantitative capillary electrophoresis for counting and quality control of RNA viruses. Anal. Chem. 84, 9585–9591 (2012).
Galasso, G. & Sharp, D. Virus particle aggregation and the plaque-forming unit. The Journal of Immunology 88, 339–347 (1962).
Kissmann, J. et al. Stabilization of measles virus for vaccine formulation. Hum. Vaccines 4, 350–359 (2008).
Das, P. Revolutionary vaccine technology breaks the cold chain. Lancet Infect. Dis. 4, 719 (2004).
Acknowledgements
We are most grateful to Dr. John C. Bell and Dr. Fabrice Le Boeuf for providing herpes virus type I and vesicular stomatitis virus expressing fluorescent proteins.
Author information
Authors and Affiliations
Contributions
S.M.G. and M.V.B. conceived the idea and designed the research. S.M.G. performed experiments with viruses. A.K.B., C.J.C. and J.G.B. synthesized carbohydrate-based ice recrystallization inhibitors and prepared figure 1. A.G. and D.M. performed capillary electrophoresis experiments. G.G.M. performed mass spectrometry experiments to study virus-small molecule binding. S.M.G. and A.K.B. wrote the paper. R.N.B. and M.V.B. edited the manuscript and supervised the study. All authors read the manuscript, provided comments and approved its content.
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Electronic supplementary material
Supplementary Information
Supplementary Info
Rights and permissions
This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder in order to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/
About this article
Cite this article
Ghobadloo, S., Balcerzak, A., Gargaun, A. et al. Carbohydrate-Based Ice Recrystallization Inhibitors Increase Infectivity and Thermostability of Viral Vectors. Sci Rep 4, 5903 (2014). https://doi.org/10.1038/srep05903
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/srep05903
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.