Abstract
The amygdala is believed to be highly relevant to the pathophysiology of obsessive–compulsive disorder (OCD) given its prominent role in fear conditioning and because it is an important target of the serotonin reuptake inhibitors (SRIs), the pharmacotherapy of choice for OCD. In the present study, we measured in vivo volumetric changes in the amygdala in pediatric patients with OCD following 16 weeks of monotherapy with the selective SRI, paroxetine hydrochloride. Amygdala volumes were computed from contiguous 1.5 mm magnetic resonance (MR) images in 11 psychotropic drug-naive patients with OCD prior to and then following treatment. Eleven healthy pediatric comparison subjects also had baseline and follow-up scans, but none of these subjects received medication. Patients demonstrated significant asymmetry of the amygdala (L>R) prior to pharmacologic intervention in contrast to healthy comparison subjects who showed no asymmetry at the time of their baseline scan. Mixed model analyses using age and total brain volume as time varying covariates indicated that left amygdala volume decreased significantly in patients following treatment. The reduction in left amygdala volume in patients correlated significantly with higher paroxetine dosage at the time of the follow-up scan and total cumulative paroxetine exposure between the scans. No significant changes in either right or left amygdala volume were evident among healthy comparison subjects from the baseline to the follow-up scan. These preliminary findings suggest that abnormal asymmetry of the amygdala may play a role in the pathogenesis of OCD and that paroxetine treatment may be associated with a reduction in amygdala volume.
Similar content being viewed by others
INTRODUCTION
Obsessive–compulsive disorder (OCD) is often characterized by chronic functional impairment and is considered the ninth leading cause of all disabilities in the United States and other countries (Murray and Lopez, 1996). The disorder appears to have a bimodal incidence with one peak occurring during childhood and the other in adulthood (Geller et al, 1998; Pauls et al, 1995). While pediatric and adult-onset OCD share a similar phenomenology and response to behavioral treatment and pharmacotherapy across the lifespan (Swedo et al, 1992), there are important differences between these forms of the disorder. In contrast to the adult form of the disorder, childhood OCD appears to have a stronger familial influence, greater male predominance, and higher incidence of tics and developmental disorders (Swedo et al, 1992; Geller et al, 1998). The investigation of neurobiological mechanisms in OCD may be facilitated by studying the illness in childhood compared to adulthood, which may decrease the likelihood of potentially confounding variables such as illness duration and prior pharmacologic exposure.
Current hypotheses with regard to neurotransmitter system abnormalities in OCD have focused on dysfunction of serotonergic systems (Cartwright and Hollander, 1998). In particular, pharmacologic studies have demonstrated the efficacy and safety of the serotonin reuptake inhibitors (SRIs) in the treatment of OCD (March et al, 1998; Rapoport et al, 1980). More direct evidence for serotonergic dysfunction in OCD, however, comes from brain imaging studies that have demonstrated reductions in brain activity in patients following the administration of SRIs (Baxter et al, 1992; Swedo et al, 1989).
The amygdala has been implicated in the pathophysiology of OCD (Szeszko et al, 1999; Rosenberg et al, 1997a) and hypothesized to represent an important neuroanatomic substrate of the anxiety that maintains compulsive behaviors in OCD (Rauch et al, 1998). The amygdala may be particularly relevant to OCD given its role in the emotional appraisal of stimuli (Bechara et al, 1995; Ketter et al, 1996; Irwin et al, 1996) and in acquiring and consolidating conditioned fear responses (Davis, 1997,1992). We suggested previously that a defect involving the ventral ‘paleocortical’ trend of brain development (Sanides, 1969), involving the amygdala and orbital frontal cortex, may represent an important neurobiological defect in the pathophysiology of OCD (Szeszko et al, 1999). Neuropsychological deficits observed on tasks of olfaction (Barnett et al, 1999) and response inhibition (Rosenberg et al, 1997b, 1997c) are consistent with the hypothesis of a defect involving ventral brain regions.
Serotonin in the amygdala has been linked with the emotional appraisal of situations (Kawahara et al, 1993), modulation of fear (Sommer et al, 2001), and conditioned anxiety (Zangrossi et al, 1999), factors that seem to play a crucial role in the phenomenology of OCD. Serotonergic receptors have been found to play a role in the inhibition of amygdala neurotransmission (Cheng et al, 1998), and the SRIs have been found to exert their effects on receptors in various nuclei within the amygdala (Nagy et al, 1979; Hodges et al, 1987; Costall et al, 1989; Gonzalez et al, 1996). Moreover, quantitative autoradiographic studies suggest that there are moderate to high densities of 3[H] paroxetine binding sites in the amygdala (Chen et al, 1992; De Souza and Kuyatt, 1987), supporting the idea that this region may be one target of pharmacologic intervention with the SRIs.
In this study, we tested the hypothesis that administration of the SRI, paroxetine hydrochloride, to patients with OCD would result in the alterations of amygdala volume as assessed from structural magnetic resonance (MR) imaging. Patients were studied early in the course of illness and psychotropic drug naive to minimize the possible confounds of illness duration and prior pharmacologic exposure on brain structure volumes.
METHODS
Subjects
In all, 11 dextral psychotropic drug-naive pediatric outpatients with OCD and 11 healthy comparison subjects participated in this study. Sample characteristics are illustrated in Table 1. Patients were ill, on average, for 1.6 years prior to study entry (SD=1.7 years, range=0.08–5.2 years). The mean age at onset was 10.2 years (SD=2.3 years, range=6.3–12.5 years). This sample does not overlap with a previous sample (Rosenberg and Keshavan, 1998; Rosenberg et al, 1997a), but includes 17 subjects reported in a more recent investigation of thalamic volume (Gilbert et al, 2000).
All patients were recruited through the child psychiatry outpatient clinic at Wayne State University School of Medicine in Detroit, Michigan. Patients were diagnosed using DSM-IV criteria (American Psychiatric Association, 1994) and the Schedule for Affective Disorders and Schizophrenia for School Age Children—Present and Lifetime (K-SADS-PL) versions (Kaufman et al, 1997). A board-certified child and adolescent psychiatrist (DRR) interviewed all subjects and their parents. The exclusion criteria for patients and healthy comparison subjects included lifetime history of unipolar or bipolar disorder, psychosis, eating disorders, substance abuse or dependence, Sydenham's chorea, Tourette syndrome, and other tic-related conditions, conduct disorder, significantly debilitating medical or neurological conditions, pervasive developmental disorders, mental retardation, or learning disorders. There was no history of psychiatric illness in healthy comparison subjects as determined from the K-SADS-PL or in any of their first-degree relatives. The child's parents served as informants. Legal guardians provided written informed consent and all subjects provided written assent.
Clinical Assessments
All subjects were administered the Children's Yale-Brown Obsessive Compulsive Scale (CYBOCS; Goodman et al, 1989; Wolff and Wolff, 1991) to assess OCD symptom severity. The 17-item Hamilton Depression Rating Scale (Hamilton, 1967) measured the severity of depression and the Hamilton Anxiety Rating Scale (Hamilton, 1959) measured the severity of anxiety. Tic severity was measured with the Yale-Global Tic Severity Scale (Leckman et al, 1989). Clinical assessment data are presented in Table 1.
MR Imaging Procedures
MR imaging exams were conducted at the Children's Hospital of Michigan Imaging Center; the image acquisition methods have been described previously (Gilbert et al, 2000). Briefly, MR images were acquired in the coronal plane using a 3D spoiled gradient echo pulse sequence with a 40° flip angle, 25 ms repetition time, and 5 ms echo time on a 1.5 T whole body superconducting imaging system (General Electric, Milwaukee, WI). This sequence produced 124 contiguous coronal slices (slice thickness=1.5 mm) through the whole head with nominal in-plane resolution of 0.94 × 0.94 mm2 in a 256 × 256 matrix. Axial proton density and T2-weighted images were obtained to exclude structural abnormalities on MR imaging scans.
Intracranial volume
National Institutes of Health image software (v1.61) (Rasband, 1996) was used to compute intracranial volume in a manner described previously (Rosenberg et al, 1997a). This technique yields valid and reliable neuroanatomical measurements using a semiautomated segmentation approach (Rasband, 1996). Inter-rater reliability between two raters (as assessed by intraclass correlations (ICCs)) in 12 cases was 0.99.
Amygdala measurement
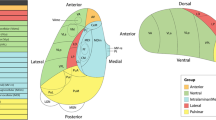
Neuroanatomical boundaries for the amygdala were based on operational criteria from post-mortem histological work (Bogerts et al, 1985) and prior published studies (Watson et al, 1992; Szeszko et al, 2003). An illustration of the amygdala boundaries is provided in Figure 1. The amygdala was measured from the slice where it first became visible posteriorly. The medial aspect of the posterior part of the amygdala was guided by the crural cistern into the transverse cerebral fissure. The superior border was defined by a straight line drawn laterally from the superiolateral aspect of the optic tract to the fundus of the circular sulcus of the insula. At its most posterior limit care was taken to exclude the tail of the caudate nucleus, globus pallidus, putamen, and the lateral geniculate body from the measurement. The medial border of the amygdala was a thin strip of white matter called the angular bundle, which separates it from the entorhinal cortex. A straight line was drawn on the angular bundle to separate the amygdala laterally from entorhinal cortex medially. The inferior and lateral borders of the amygdala were formed from the temporal horn or adjacent white matter. More anteriorly, the superior border of the amygdala was defined by drawing a straight line from the inferior portion of the circular sulcus of the insula to the endorhinal sulcus. The anterior boundary was the slice where the amygdala no longer appeared to have an ovoid shape, which was either at or posterior to the slice where the closure of the lateral sulcus formed the endorhinal sulcus.
Measurement of the amygdala was completed in MEDx (Medx, 1998) following alignment along the anterior and posterior commissures for purposes of standardization. Prior to measurements, scans of patients and comparison subjects were flipped randomly in the right–left axis and mixed together. No identifying information was available from the scan. All measurements were thus completed by a single well-trained and reliable operator (SM) who was blind to group membership, hemisphere, and scan time point. Right amygdala volume could not be computed for one healthy volunteer at the time of the baseline scan due to scan artifact. ICCs for the right and left amygdala were 0.85 and 0.93, respectively.
Paroxetine Treatment
Paroxetine treatment began at 10 mg/day for all patients and was titrated to a maximum dosage of 60 mg/day based on response (mean (SD)=38 mg/day; SD=16; range=10–60 mg/day). Patients with OCD had follow-up clinical assessments and MR imaging exams after receiving paroxetine hydrochloride for an average of 16 weeks (SD=3.8). Patients were monitored for medication side effects and adverse experiences during the treatment trial. All patients received paroxetine only and were not receiving cognitive-behavioral therapy or psychotherapy other than supportive therapy. Patients did not participate in this part of the study if: (1) they were unable to be maintained on paroxetine monotherapy; (2) there was a contraindication to paroxetine therapy; (3) there was a need for additional behavioral or psychosocial interventions, or (4) the patient's parents refused to consent to their child taking psychotropic medications, MR imaging procedures, and/or the treating psychiatrist determined that alternative treatment was warranted.
Data Analysis
Group differences in clinical assessments and intracranial volume were examined using independent groups t-tests. χ2 tests were used to examine group differences in sex, handedness, and social class. Paired t-tests were used to compare pretreatment and post-treatment intracranial volume, CYBOCS, Hamilton Depression Rating Scale, Hamilton Anxiety Rating Scale, and Yale Global Tic Severity Scale scores in the patient group. Tests of association between continuous variables were examined using Pearson's product moment correlations.
Mixed models analyses (SAS, 2001) were used to examine group differences in amygdala volume using age and total intracranial volume as time-dependent covariates. Thus, changes in amygdala volume were examined while controlling for changes in age and intracranial volume between the scans. Age was included as a statistical covariate given that patients tended to be younger than healthy comparison subjects. Intracranial volume was included as a statistical covariate to control for nonspecific changes in amygdala volume over time. In addition, given group differences in height and weight, we investigated the potential effects of these variables on amygdala volumes by including them as statistical covariates in subsequent analyses. The group (patient vs healthy comparison subject) was a between-subject factor and time (initial vs follow-up scan) and hemisphere were within-subject factors. Right and left hemisphere volumes could not be pooled in the analyses because preliminary analyses revealed a significant hemisphere-by-time interaction for the patient group. To examine whether changes in brain structure volumes were associated with changes in symptoms or medication dosage, we computed percent change scores relative to baseline scores [((Post-test−pretest)/(pretest)) × 100]. All analyses were two-tailed with alpha set to 0.05.
RESULTS
Sample characteristics for patients and healthy volunteers are provided in Table 1. Patients did not differ significantly from healthy comparison subjects in distributions of age, height, sex, or parental social class (ps>0.05). Patients, however, weighed significantly less compared to healthy volunteers. Patients did not differ significantly from healthy volunteers in intracranial volume either at the time of the baseline or follow-up scans (ps>0.05).
Following treatment with paroxetine, patients showed significant reductions in symptom severity as reflected by their lower CYBOCS (t1,10=4.37, p=0.001; pretreatment=28.5; SD=6.4 and post-treatment=18.2; SD=7.9), Hamilton Depression (t1,10=2.71, p=0.02; pretreatment=10.0; SD=5.5 and post-treatment=3.9; SD=3.5) and Hamilton Anxiety (t1,10=2.63, p=0.03; pretreatment=10.4; SD=7.2 and post-treatment=4.0; SD=2.3) scores. Illness duration was not significantly correlated with either right or left amygdala volumes at the time of the baseline scan (ps>0.05).
While controlling for changes in age and intracranial volume between scans, the main finding that distinguished patients from healthy comparison subjects for amygdala volume was a significant group-by-hemisphere-by-time interaction (F1,56=2.67, p=0.02). At the time of the initial scan patients had significantly larger left amygdala volume compared to their right amygdala volumes (t1,56=3.00, p=0.004; see Figure 2); this asymmetry was absent among healthy comparison subjects (see Figure 3). Patients did not differ from healthy comparison subjects in either right or left baseline amygdala volumes (ps>0.05). Following the administration of paroxetine patients showed a significant reduction in left amygdala volume (t1,56=3.26, p=0.002; see Figure 2), which decreased, on an average, by 0.25 cm3 or approximately 15%. The reductions in left amygdala volume occurred in nine of 11 children (see Figure 4). Among healthy comparison subjects there was no significant change in left amygdala volume from the initial scan to the follow-up scan (p>0.05; see Figure 3). The use of height and weight as statistical covariates and the exclusion of the one nondextral participant from analyses did not alter these findings.
Among patients, the reduction in left amygdala volume did not correlate significantly with changes in clinical measures (ps>0.05), but correlated significantly with higher paroxetine dosage at the time of the follow-up scan (r=−0.65, N=11, p=0.03) and total cumulative paroxetine exposure received between the scans (r=−0.71, N=11, p=0.01).
These correlations remained statistically significant while controlling for changes in clinical symptoms. At the time of the follow-up scan neither patients nor healthy comparison subjects demonstrated significant asymmetry in amygdala volume (ps>0.05; see Figures 2 and 3), and there were no significant group differences in either right or left amygdala volume (ps>0.05).
DISCUSSION
To our knowledge this study represents the first in vivo demonstration of changes in amygdala volume in OCD following SRI pharmacotherapy using structural MR imaging. The main findings were that pediatric psychotropic drug-naïve patients with OCD demonstrate greater left than right amygdala volume compared to healthy volunteers and that the administration of paroxetine to patients was associated with a reduction in left amygdala volume in a dose-dependent manner. The reduction in volume remained significant after controlling for changes in total intracranial volume and age, and was in contrast to the lack of significant change in amygdala volume in untreated healthy comparison children.
The finding of larger left compared to right amygdala volume in our sample of medication-free patients is consistent with the results of Kwon et al (2003), who reported significantly larger left amygdala volume in their sample of OCD patients compared to patients with schizophrenia and healthy volunteers. These findings may have relevance for a prior study that reported smaller amygdala volume in previously treated adults with OCD compared to healthy comparison subjects (Szeszko et al, 1999). It is possible that in our prior study smaller amygdala volume was associated with treatment. It is difficult to compare these studies directly; however, given differences in the populations, imaging methodology, and methods for measuring the amygdala. For example, in our prior study measurements of the amygdala included the most rostral part of the anterior hippocampus. Nevertheless, our findings do highlight the importance of controlling for prior medication history when examining volumetric measures of the brain anatomy.
There may be several possible mechanisms through which paroxetine could result in amygdala volume reductions in our patient sample. Although disease progression cannot be entirely ruled out, this seems unlikely given the magnitude of the change and brief duration in which these changes occurred. In addition, therapeutic doses of paroxetine appear to lack any important hemodynamic or electrophysiological effects (Warrington and Lewis, 1992), thus arguing against these possibilities. It seems plausible that the reductions in amygdala volume were treatment induced given the significant inverse relationship between these reductions and paroxetine treatment. Specifically, paroxetine treatment in OCD may be associated with a reversal of left amygdala hypertrophy given that right and left amygdala volumes were comparable following treatment. It is possible that SRI administration results in deactivation and consequent hypotrophy of neural components within the amygdala. In addition, downregulation of serotonergic receptors following paroxetine administration has also been reported in young, depressed patients (Meyer et al, 2001), and this could also conceivably be associated with a reduction in amygdala volume. Whether such volumetric alterations are plastic over the course of treatment could not be addressed in the present study, but would be an important question for future research.
Our findings are consistent with studies demonstrating an inhibitory effect of SRIs on brain structure and function. The SRIs appear to target various amygdaloid nuclei (Costall et al, 1989; Gonzalez et al, 1996) and inhibition of neurotransmission within the amygdala may be mediated partly by serotonergic receptors (Cheng et al, 1998). In addition, animal studies indicate that administration of SRIs is associated with decreased stress-induced Fos-like immunoreactivity in the medial amygdala (Lino-de-Oliveira et al, 2001). Functional neuroimaging studies reported decreased cortical and subcortical glucose metabolism in patients with OCD following SRI administration (Baxter et al, 1992; Swedo et al, 1989) and normalization of left amygdala hyperarousal was identified in patients with major depressive disorder after SRI pharmacotherapy (Sheline et al, 2001). The present findings also converge with our prior study demonstrating thalamus volume reductions in OCD following SRI pharmacotherapy (Gilbert et al, 2000), suggesting that paroxetine may be exerting similar inhibitory effects on other brain regions implicated in the pathophysiology of OCD. In this regard, it is worth noting that no significant changes in intracranial volume were observed in patients following treatment, suggesting that our findings were not an artifact of global volumetric reductions. The potential inhibitory effects of the SRIs may depend on the underlying pathophysiology of the disorder (Saxena et al, 2002) however, and thus, our findings may not be generalizable to other clinical populations. In addition, although prior studies reported neurogenesis in humans (Vermetten et al, 2003) and rats (Malberg et al, 2000) following SRI pharmacotherapy, these effects were observed in the hippocampus, and thus may not be directly comparable to our findings.
The finding that changes in amygdala volume following paroxetine administration were lateralized to the left hemisphere is consistent with prior studies implicating dysregulation of left hemisphere cortical networks in OCD (Baxter et al, 1987; Brody et al, 1998). In a study of correlations between normalized regional cerebral metabolic rates for glucose, Horwitz et al (1991) found that the left hemisphere anterior medial temporal region (which mainly included the amygdala) was one of the regions that had the largest number of reference ratio correlations that differed significantly between patients with OCD and healthy comparison subjects. Moreover, the left anterior medial temporal region had increased interactions with left frontal structures in OCD relative to healthy individuals. It is also worth noting that the left amygdala has been reported to play a role in anticipatory anxiety (Phelps et al, 2001) and responds when individuals are conscious of the aversive nature of a stimulus (Morris et al, 1998), factors which appear to be highly relevant to the phenomenology of OCD.
There were several limitations to this study that preclude firm conclusions. The number of participants was small, and thus these findings should be considered preliminary until replicated in a larger sample. Although a double-blind placebo-controlled study may have been superior for delineating treatment vs placebo effects, such a study would have required a much larger sample and been more difficult to justify given the efficacy of the SRIs compared to placebo in treating OCD. Another potential study limitation was that changes in amygdala volume did not correlate significantly with symptom changes. This lack of association might reflect the possibility that symptom changes occurred at a differential rate compared to volumetric changes. Also, given that the amygdala maintains connections with other serotonergic regions implicated in the pathophysiology of OCD such as the thalamus (Price, 2003; Oke et al, 1997), it is possible that the observed amygdala volumetric changes are an epiphenomena of the underlying pathophysiology of the disorder and/or a nonspecific correlate of treatment intervention. Moreover, changes in the dopaminergic system could also be related to the observed volumetric alterations given that animal studies have demonstrated that dopamine inhibition may be indirectly affected by serotonin (Korsgaard et al, 1985).
In summary, our results suggest that an abnormality involving amygdala asymmetry may play a role in the pathogenesis of OCD and that reductions in amygdala volume are associated with paroxetine treatment. Further studies are needed to assess the potential long-term neuroanatomical changes of the SRIs and mechanisms of response.
References
American Psychiatric Association (1994). Diagnostic and Statistical Manual of Mental Disorders, 4th edn American Psychiatric Association: Washington, DC.
Barnett R, Maruff P, Purcell R, Wainwright K, Kyrios M, Brewer W et al (1999). Impairment of olfactory identification in obsessive–compulsive disorder. Psychol Med 29: 1227–1233.
Baxter LR, Phelps ME, Mazziotta JC, Guze BH, Schwartz JM, Selin CE (1987). Local cerebral glucose metabolic rates in obsessive–compulsive disorder: a comparison with rates in unipolar depression and normal controls. Arch Gen Psychiatry 44: 211–218.
Baxter LR, Schwartz JM, Bergman KS, Szuba MP, Guze BH, Mazziotta JC et al (1992). Caudate glucose metabolite changes with both drug and behavior therapy for obsessive–compulsive disorder. Arch Gen Psychiatry 49: 681–689.
Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR (1995). Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science 269: 1115–1118.
Bogerts B, Meertz E, Schonfeld-Bausch R (1985). Basal ganglia and limbic system pathology in schizophrenia. Arch Gen Psychiatry 42: 784–791.
Brody AL, Saxena S, Schwartz JM, Stoessel PS, Maidment K, Phelps ME (1998). FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Res 84: 1–6.
Cartwright C, Hollander E (1998). SSRls in the treatment of obsessive-compulsive disorder. Depress Anxiety 8: 105–113.
Chen HT, Clark M, Goldman D (1992). Quantitative autoradiography of 3H-paroxetine binding sites in rat brain. J Pharmacol Toxicol Methods. 27: 209–216.
Cheng LL, Wang SJ, Gean PS (1998). Serotonin depresses excitatory synaptic transmission and depolarization-evoked Ca2+ influx in rat basolateral amygdala via 5-HT1A receptors. Eur J Neurosci 10: 2163–2172.
Costall B, Kelly ME, Naylor RJ, Onaivi ES, Tyers MB (1989). Neuroanatomical sites olfaction of 5-HT-3 receptor agonist and antagonists for alteration of aversive behavior in the mouse. Br J Pharmacol 96: 325–332.
Davis M (1992). The role of the amygdala in fear and anxiety. Annu Rev Neurosci 15: 353–375.
Davis M (1997). Neurobiology of fear responses: the role of the amygdala. J Neuropsychiatry Clin Neurosci 9: 382–402.
De Souza EB, Kuyatt BL (1987). Autoradiographic localization of 3H-paroxetine-labeled serotonin uptake sites in rat brain. Synapse 1: 488–496.
Geller DA, Biederman J, Jones J, Shapiro S, Schwartz S, Park KS (1998). Obsessive–compulsive disorder in children and adolescents: a review. Harv Rev Psychiatry 5: 260–273.
Gilbert AR, Moore GJ, Keshavan MS, Paulson LA, Narula V, MacMaster FP et al (2000). Decrease in thalamic volumes of pediatric patients with obsessive–compulsive disorder who are taking paroxetine. Arch Gen Psychiatry 57: 449–456.
Gonzalez LE, Andrews N, Files SE (1996). 5-HT1A and benzodiazepine receptors in the basolateral amygdala module anxiety in the social interaction test, but not in the elevated plus-maze. Brain Res 732: 145–153.
Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL et al (1989). The Yale-Brown Obsessive Compulsive Scale I: development, use and reliability. Arch Gen Psychiatry 46: 1006–1011.
Hamilton M (1967). Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6: 278–296.
Hamilton M (1959). The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55.
Hodges H, Green S, Glenn B (1987). Evidence that the amygdala is involved in benzodiazepine and serotonergic effects on punished responding but not on discrimination. Psychopharmacology 92: 491–504.
Horwitz B, Swedo SE, Grady CL, Pietrini P, Schapiro MB, Rapoport JL et al (1991). Cerebral metabolic pattern in obsessive–compulsive disorder: altered interconnections between regional rates of glucose utilization. Psychiatry Res 40: 221–237.
Irwin W, Davidson RJ, Lowe MJ, Mock BJ, Sorenson JA, Turski PA (1996). Human amygdala activation detected with echo-planar functional magnetic resonance imaging. Neuroreport 7: 1765–1769.
Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P et al (1997). Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36: 980–988.
Kawahara H, Yoshida M, Yokoo H, Nishi M, Tanaka M (1993). Psychological stress increases serotonin release in the rat amygdala and prefrontal cortex assessed by in vivo microdialysis. Neurosci Lett 162: 81–84.
Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI et al (1996). Anterior paralimbic mediation of procaine-induce emotional and psychosensory experiences. Arch Gen Psychiatry 53: 59–69.
Korsgaard S, Gerlach J, Christensson E (1985). Behavioural aspects of serotonin–dopamine interaction in monkey. Eur J Pharmacol 38: 118:245–118:252.
Kwon JS, Shin YW, Kim CW, Kim YI, Youn T, Han MH et al (2003). Similarity and disparity of obsessive–compulsive disorder and schizophrenia in MR volumetric abnormalities of the hippocampus-amygdala complex. J Neurol Neurosurg Psychiatry 74: 962–964.
Leckman JF, Riddle MA, Hardin MT, Ort SL, Swartz KL, Stevenson J et al (1989). The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry 28: 566–577.
Lino-de-Oliveira C, Sales AJ, Del Bel EA, Silveira MC, Guimaraes FS (2001). Effects of acute and chronic fluoxetine treatments on restraint stress-induces Fos expression. Brain Res Bull 55: 747–754.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS (2000). Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci 20: 9104–9110.
March JS, Biederman J, Wolkow R, Safferman A, Mardekian J, Cook E et al (1998). Sertraline in children and adolescents with obsessive-compulsive disorder: a multicenter randomized controlled trial. JAMA 280: 1752–1756.
Medx (1998). Sensor Systems, Inc.: Sterling, Virginia.
Meyer JH, Kapur S, Eisfeld B, Brown GM, Houle S, DaSilva J et al (2001). The effect of paroxetine on 5-HT(2A) receptors in depression: an [(18)F]setoperone PET imaging study. Am J Psychiatry 158: 78–85.
Morris JS, Ohman A, Dolan RJ (1998). Conscious and unconscious emotional learning in the human amygdala. Nature 393: 467–470.
Murray CJL, Lopez AD (1996). The Global Burden of Disease. Harvard University Press: Boston, MA.
Nagy J, Zambo K, Decsi L (1979). Anti-anxiety action of diazepam after intra-amygdaloid application in the rat. Neuropharmacology 18: 573–576.
Oke AF, Carver LA, Gouvion CM, Adams RN (1997). Three-dimensional mapping of norepinephrine and serotonin in human thalamus. Brain Res 763: 69–78.
Pauls DL, Alsobrook II JP, Goodman W, Rasmussen S, Leckman JF (1995). A family study of obsessive–compulsive disorder. Am J Psychiatry 152: 76–84.
Phelps EA, O'Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M (2001). Activation of the left amygdala to a cognitive representation of fear. Nat Neurosci 4: 437–441.
Price JL (2003). Comparative aspects of amygdala connectivity. Ann NY Acad Sci 985: 50–58.
Rapoport J, Elkins R, Mikkelsen E (1980). Clinical trial of chlomipramine in adolescents with obsessive–compulsive disorder. Psychopharmacol Bull 16: 61–63.
Rasband W (1996). National Institutes of Health Image Manual. ational Institutes of Health: Bethesda, MD.
Rauch SL, Whalen PJ, Dougherty D, Jenike MA (1998). Neurobiological models of obsessive–compulsive disorder. In: Jenike MA, Baer L, Minichiello WE (eds) Obsessive–compulsive Disorders Practical Management. : Mosby Inc.: St Louis, MO. pp 222–253.
Rosenberg DR, Averbach DH, O'Hearn KM, Seymour AB, Birmaher B, Sweeney JA (1997b). Oculomotor response inhibition abnormalities in pediatric obsessive–compulsive disorder. Arch Gen Psychiatry 54: 831–838.
Rosenberg DR, Dick EL, O'Hearn KM, Sweeney JA (1997c). Response-inhibition deficits in obsessive–compulsive disorder: dysfunction in frontostriatal circuits. J Psychiatry Neurosci 22: 29–38.
Rosenberg DR, Keshavan MS, O'Hearn KM, Dick EL, Bagwell WW, Seymour AB et al (1997a). Fronto-striatal measurement of treatment-naive pediatric obsessive–compulsive disorder. Arch Gen Psychiatry 54: 824–830.
Rosenberg DR, Keshavan MS (1998). A.E. Bennett Research Award. Toward a neurodevelopmental model of obsessive–compulsive disorder. Biol Psychiatry 43: 623–640.
Sanides F (1969). Comparative architectonics of the neocortex of mammals and their evolutionary interpretation. Ann NY Acad Sci 167: 404–423.
Statistical Analysis Software, v 8.2 (2001). SAS Institute Inc.: Cary, North Carolina.
Saxena S, Brody AL, Ho ML, Alborzian S, Maidment KM, Zohrabi N et al (2002). Differential cerebral metabolic changes with paroxetine treatment of obsessive–compulsive disorder vs major depression. Arch Gen Psychiatry 59: 250–261.
Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA (2001). Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry 50: 651–658.
Sommer W, Moller C, Wiklund L, Thorsell A, Rimondini R, Nissbrandt H et al (2001). Local 5, 7-dihydroxtyptamine lesions of rat amygdala: release of punished drinking, unaffected plus-maze behavior and ethanol consumption. Neuropsychopharmacology 24: 430–440.
Swedo SE, Leonard HL, Rapoport JL (1992). Childhood-onset obsessive–compulsive disorder. Psychiatr Clin N Am 15: 767–775.
Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A et al (1989). Cerebral glucose metabolism in childhood obsessive–compulsive disorder. Arch Gen Psychiatry 46: 518–523.
Szeszko PR, Goldberg E, Gunduz-Bruce H, Ashtari M, Robinson D, Malhotra AK et al (2003). Smaller volume of the anterior hippocampal formation in antipsychotic drug-naïve patients with first-episode schizophrenia. Am J Psychiatry 160: 2190–2197.
Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M et al (1999). Orbital frontal and amygdala volume reductions in obsessive–compulsive disorder. Arch Gen Psychiatry 56: 913–919.
Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD (2003). Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 54: 693–702.
Warrington SJ, Lewis Y (1992). Cardiovascular effects of antidepressants: studies of paroxetine in healthy men and depressed patients. Int Clin Psychopharmacol 6: 59–64.
Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A et al (1992). Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 42: 1743–1750.
Wolff RP, Wolff LS (1991). Assessment and treatment of obsessive–compulsive disorder in children. Behav Modif 15: 372–393.
Zangrossi Jr H, Viana MB, Graeff FG (1999). Anxiolytic effect of intra-amygdala injection of midazolam and 8-hydroxy-2-(di-n-propylamino) tetralin in the elevated T-maze. Eur J Pharmacol 369: 267–270.
Acknowledgements
This work was supported in part by grants from NARSAD (PRS, DRR) and the National Institute of Mental Health to Dr Szeszko (MH01990) and Dr Rosenberg (MH59299; MH01372; MH02037; MH65122) as well as the Miriam L Hamburger Endowed Chair at the Children's Hospital of Michigan and Wayne State University, Detroit, MI (DRR) and the State of Michigan Joe F Young Sr Psychiatric Research and Training Program. A previous version of this study was presented at the 2001 Meeting of the American College of Neuropsychopharmacology in Waikoloa, Hawaii.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Szeszko, P., MacMillan, S., McMeniman, M. et al. Amygdala Volume Reductions in Pediatric Patients with Obsessive–Compulsive Disorder Treated with Paroxetine: Preliminary Findings. Neuropsychopharmacol 29, 826–832 (2004). https://doi.org/10.1038/sj.npp.1300399
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.npp.1300399
Keywords
This article is cited by
-
Mapping the neuroanatomical abnormalities in a phenotype of male compulsive rats
Behavioral and Brain Functions (2023)
-
Decreased left amygdala functional connectivity by cognitive-coping therapy in obsessive-compulsive disorder
Molecular Psychiatry (2021)
-
Bigger is better! Hippocampal volume and declarative memory performance in healthy young men
Brain Structure and Function (2014)
-
Neurochemicals measured by 1H-MR spectroscopy: putative vulnerability biomarkers for obsessive compulsive disorder
Magnetic Resonance Materials in Physics, Biology and Medicine (2014)
-
Disorder-specific volumetric brain difference in adolescent major depressive disorder and bipolar depression
Brain Imaging and Behavior (2014)