ABSTRACT
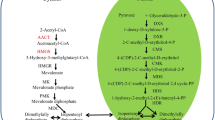
Plants synthesize the osmoprotectant glycine betaine (GB) via choline→ betaine aldehyde→glycine betaine1. Two enzymes are involved in the pathway, choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH). A full length CMO cDNA (1,643bp) was cloned from Amaranthus tricolor. The open reading frame encoded a 442-amino acid polypeptide, which showed 69% identity with CMOs in Spinacia oleracea L. and Beta vulgaris L. DNA gel blot analysis indicated the presence of one copy of CMO gene in the A. tricolor genome. The expressions of CMO and BADH proteins in A.tricolor leaves significantly increased under salinization, drought and heat stress (42°C), as determined by immunoblot analysis, but did not respond to cold stress (4°C), or exogenous ABA application. The increase of GB content in leaves was parallel to CMO and BADH contents.
Similar content being viewed by others
INTRODUCTION
Amaranth is a C4 dicotyledonous mesophyte crop plant. A. tricolor is a major variety for vegetable and ornamental crops, and is widely cultivated in the world. Osmoprotectant glycine betaine (GB) was detected in Amaranthaceae, A. Hypochondriacus L2 and A. Caudatus L3, 4. GB is widespread and an effective osmoprotectant in many plants3. We studied the photosynthetic adaptation mechanism of A. tricolor under salt stress due to accumulation of GB5.
GB is synthesized by the two-step oxidation of choline. The first step is catalyzed by choline monooxygenase (CMO), which has only been studied in Chenopodiaceae, spinach and sugar beet up to now. Afterward cDNAs encoding CMO proteins were cloned from both species4, 6, and the deduced amino acid sequences showed 84% identity. Spinach CMO is a stromal, Fd-dependent monooxygenase with a Rieske-type [2Fe-2S] center7, and is completely different from the bacterial choline dehydrogenase and oxidase enzymes. CMO was assumed to be unique among plant oxygenases6, 8. The second step of GB synthesis is mediated by betaine aldehyde dehydrogenase (BADH)1, which has been well documented in amaranth2, 9 and other plants10, 11, 12, 13, 14.
CMO and BADH expressions in spinach leaves, and sugar beet leaves and taproots4, 6, 7 were regulated by salt and drought stresses. A. caudatus L. in addition to Chenopodiaceae was first known to accumulate GB and express CMO protein under salt stress5.
In present work, we investigated CMO proteins in GB-accumulated species of Chenopodiaceae and Amaranthaceae. Furthermore, we examined CMO, BADH proteins expression and GB contents in A. tricolor under conditions of environmental stresses including salt, drought, cold, heat, and exogenous ABA application. We also isolated the CMO cDNA from A. tricolor cDNA library.
MATERIALS AND METHODS
A. tricolor, A. caudatus, A. hypochondriacus, Spinacia oleracea, Suaeda japonica and Atriplex ssp.gmelini seeds were germinated and their seedlings grown under the conditions described by Wang et al5. 6-week-old A. tricolor plants were exposed to the following treatments, respectively: 300 mM NaCl for 5 d, drought by withholding water for 10 d, cold (4°C) for 8 d, and heat stress (42°C) for 3 d. 100 mm ABA in water was sprayed on the surface of all leaves until solution runoff, repeated spreading three times, the plants further grew 5 d. Measurements of protein and GB were carried out as previously described5.
cDNA cloning
Total RNA from salinized A. tricolor leaves was extracted as described by Yamamoto et al15. A 947-bp DNA fragment was generated using reverse transcription-PCR with degenerate primers. The reverse and forward primers were: 5′-TCYAARGGNTGGCARGTNGCNGG-3′; and 5′-CCARCARTGRAARTGRTGDATNCC-3′, respectively. Poly(A) RNA was isolated using PolyATtract Series 9600™ mRNA isolation system (Promega, USA), and used to construct a cDNA library (1×106 plaque-forming units) in UniZap XR (Stratagene, USA). The specific primers were synthesized for screening the cDNA library. The forward primer and reverse primer were: 5-GATGGCAAGTCGCAGGATATAG-3 and 5′-AGCAATGGAAATGATGGATTCCT-3′, respectively. The library was screened using a PCR-96-well plate method as previously described16. DNA sequences were determined with a BigDye Terminator Cycle Sequencing ready Reaction Kit (PE-ABI, Warrighton, UK). Sequence was analyzed using PC-GENE (TM IntelliGentics Inc. Switzerland).
DNA gel blot analysis
DNA was isolated from the leaves of A. tricolor according to the method described by Chen et al17. DNA was cleaved and denatured in the gel and transferred to Hybond-N+ membrane (Amersham, UK). A 1308-bp fragment of CMO cDNA digested by BamH I and Xba I, was used as a probe. A standard protocol was then used for DNA hybridization18.
SDS-PAGE, native PAGE and immunoblot analysis
Rabbit antisera were raised against BADH and CMO as previously described5. To determine both proteins expression under various stresses, total proteins were extracted from A. tricolor leaves, according to an established method5, and were separated by 12.5% SDS-PAGE. To compare molecular weight of CMO and its subunits between A. tricolor and spinach, the proteins were extracted from salinized leaves in previously described buffer5 supplemented with 10-25 mM PMSF and 1-10 mM NEM, then separated by 12.5% SDS-PAGE or 10% native PAGE, and transferred to nitrocellulose membrane (Amersham, UK).
RESULTS
Isolation of choline monooxygenase cDNA clone
A cDNA library was prepared using mRNA from salinized A. tricolor leaves, and screened with PCR methods16. A full-length cDNA of 1,643 bp encoding a 442-amino acid polypeptide was obtained. This cDNA has 5′ and 3′ untranslated region of 54 bp and 260 bp. The DNA and deduced amino acid sequence are shown in Fig 1. When the nucleotide sequence and deduced amino acid sequence were aligned with those of spinach and sugar beet CMOs, their identities attained 73.6% and 75.7% at nucleotide level; 69.4% and 69.5% at amino acid level, respectively. Comparison of the N-terminal region of A. tricolor CMO with those of sugar beet and spinach suggests that they are very similar and share typical amino acid composition (Fig 2). The amino acid sequence of A. tricolor CMO possesses characteristics of transit peptide which target polypeptide to chloroplast19. A. tricolor CMO clone shares two consensus sequences with spinach6 and sugar beet CMO4: one is Cys-X-His-X15-17-Cys-X-X-His which coordinates with a Rieske-type [2Fe-2S] cluster. The other is a sequence containing coordination sites for mononuclear nonheme Fe: Glu/Asp-X3-4-Asp-X2-His-X4-5-His20, 21.
Nucleotide and deduced amino acid sequences of CMO cDNA from A. tricolor. Single-Rieske-type iron-sulfur [2Fe-2S] cluster-binding region is underlined, boxes mark the conserved Cys-His pairs residues. The conserved residues of the mononuclear Fe-binding motif are double underlined, and the gray boxes indicate the conserved residues. The asterisk shows processing site of N-terminal residue corresponding to the mature CMO polypeptide of spinach6. The accession number for A. tricolor CMO nucleotide sequence is AF290974.
Comparison of the deduced of 5′-transit peptide amino acid sequences of CMOs from A. tricolor, Spinacia oleracea L.6 and Beta vulgaris L.4. The gray boxes indicate identical amino acids. The asterisk indicates processing site of the N-terminal residue corresponding to the mature CMO polypeptide of spinach6. Number on the right refer to amino acid residues.
Genomic DNA Gel Blot analysis
A.tricolor genomic DNA was digested with BamH I, EcoR I, Hind III and Xba I. Blot analysis revealed single bands which represent the BamH I, EcoR I and Xba I digestion fragments, respectively, and two bands representing the Hind III digestion fragment (Fig 3a). A restriction endonuclease cleavage sites of CMO cDNA indicates that there are two Hind III sites, one at 743 and the other at 1430 bp (Fig 3b). This revealed that the CMO gene contained an intron(s) between the two Hind III sites, and one copy of CMO gene was present within A. tricolor genome.
(A)DNA gel blot analysis of A. tricolor genomic DNA. 10mg genomic DNA was digested with Bam H I, Eco R I, Hind III and Xba I, respectively, separated on 1% agarose gel, transferred to a Hybond-N+ membrane, and hybridized with a 32P-labeled CMO cDNA probe. The probe was a 1308 bp fragment digested by Bam H I- Xba I. Lanes are as follows: B, Bam H I; E, Eco R I; H, Hind III and X, Xba I. Numbers on the right are the molecular weight markers of λ-DNA digested with Hind III in kb. (B) The full length of A. tricolor cDNA and its partial restriction map. The bold lines indicate the cDNA, double lines are the vector DNA. The numbers indicate the sites of restriction endonuclease cleavage in the cDNA. Restriction cleavage site: B, Bam H I; E, EcoR I; H, Hind III and X, Xba I.
CMOs in different species
We examined CMO protein in several species of Amaranthaceae and Chenopodiaceae by immunoblot analysis. CMO proteins could be detected and showed subunits in different molecular weights (Fig 4A and 4B). The subunit of A. tricolor CMO had a lower molecular weight than that of the other two Amaranthaceae plants, A. caudatus L. and A. hypochondriacus L. (Fig 4A). Spinach CMO subunits showed higher molecular weight in three species of Chenopodiaceae, Spinacia oleracea L. Suaeda japonica and Atriplex ssp. Gmelini (Fig 4B). Meanwhile we compared the subunits of A. tricolor and spinach CMOs, and found the molecular weight of A. tricolor CMO subunit was lower than that of spinach CMOs. On the contrary, the results of native PAGE showed that the molecular weight of A. tricolor CMO was higher than that of spinach (Fig 5).
Immunoblot analysis of CMO. Total proteins (100 μg) from leaves were separated by SDS-PAGE and immunoblotted using anti-CMO antibodies. The plants were treated with 300mM NaCl for 5 d. Lanes are as follows: (A) A. tricolor (lane 1), A. caudatus L. (lane 2), A. hypochondriacus L. (lane 3). (B) Spinach ( Spinacia oleracea L.) (lane 1), Suaeda japonica (lane 2), Atriplex ssp. Gmelini (lane 3), A. tricolor (lane 4).
Immunoblot analysis of CMO and its subunit. Total proteins (100 μg) from leaves were separated by SDS-PAGE (A) and Native gel (B) followed by immunoblotting with anti-CMO antibodies. The six-week old seedlings of A. tricolor (a) and Spinacia oleracea L. (s) were treated with 300 m M NaCl for 5 d. The reported molecular weights were averages of 3-5 replicate measurements.
CMO expression under stress conditions
In order to obtain more information about the regulation of CMO expression, the effects of several types of abiotic stresses on the CMO and BADH expressions were assessed by immunoblot analysis. The results showed that the expression of CMO protein increased significantly by salt, drought and heat stress (Fig 6A). A maximum response was observed in the heat stressed leaves, where 3 d after treatment, the content of CMO protein was the highest among that under other treatments. Compared with the changes of CMO contents, the changes of BAD contents were not so significant. The basal amount of BADH protein was higher in non-stressed plants. The highest levels of GB were also detected in heat stress (Fig 6C), followed by salt and drought stresses, accompanied by an altered expression pattern of CMO and BADH proteins (Fig 6A, 6B). Such changes were not observed in cold and ABA treatment.
CMO (A), BADH (B) and glycine betaine (C) accumulation in A. tricolor under several abiotic stresses. Equal amounts (100 μg for CMO, 50 μg for BADH) of total protein were run on SDS-PAGE and immunoblotted with anti-CMO and anti-BADH antibodies. Control plants (lane 1), and plants under salt stress induced by 300 m M NaCl for 5 d (lane 2), drought stress for 10 d (lane 3), 100m M ABA treatment for 5 d (lane 4), cold treatment (4°C) for 8 d (lane 5), and 42°C heat stress for 3 d (lane 6) were studied. GB contents were average of 5 replicate measurements.
DISCUSSION
CMO protein was detected in A. tricolor, and its cDNA was isolated from A. tricolor in addition to Chenopodiaceae family. Since Amaranthaceae are phylogenetically close to Chenopodiaceae, the nucleotide and deduced amino acid sequences of their CMO reveal a high identity with spinach and sugar beet CMOs. Spinach and sugar beet CMOs have 60 and 65-residues which target the stroma4. Through judging from the processing sites of spinach and sugar beet, A. tricolor might have a transit peptide of 58 residues.
The results of SDS-PAGE and native PAGE implied that A. tricolor CMO consisted of subunits(Fig 5). The quaternary structure of CMO is not clear. Spinach CMO might be a homodimer consisting of subunits around 43 kDa, but the possibility of additional subunits cannot be ruled out7, 8. Although the deduced amino acid sequence of A. tricolor CMO cDNA is similar to that of spinach in molecular weight, its subunit weighed less than spinach CMO subunit from this study (Fig 5). When PMSF and NEM were added into the extracting buffer in order to prevent protein denaturation and degradation, the same results reappeared. Molecular weight difference of CMO subunits in several species of Amaranthaceae and Chenopodiaceae implied a possibility of different processing sites among CMO precursors (Fig 4). Because of the low amount of CMO in A. tricolor, determination of the processing site and subunit composition of its CMO was hindered.
In this study, the expressions of CMO and BADH in A.tricolor under heat stress, salt and drought stresses (Fig 6), showed that both expressions of CMO and BADH were inducible. These results indicated that the enzymes involved in GB synthesis increase by de novo protein synthesis under stress conditions. Compared with the changes of CMO contents, the changes of BADH contents were not so significant. BADHs were not a substrate-specific enzyme that had side effects of polyamine and 3-dimethylsulfonylpropionate metabolism than it did on altering the GB pathway itself22, 23. The present results and those of an additional study (to be published elsewhere) suggest that CMO plays a key role and is directly involved in GB synthesis in A. tricolor.
Abscisic acid (ABA) mediates desiccation tolerance in plants, and is involved in their response to other abiotic stress such as salt, cold, and heat24. It is well documented that ABA is an essential mediator in plant response to environmental stresses25. ABA and drought treatments can trigger an increase in BADH mRNA levels and the content of BADH protein in barley leaves at low temperature, but the results showed a low level accumulation of GB12, 26. Naidu et al27 reported that concentration of accumulated betaine in cold-stressed wheat seedlings increased more than doubled in the controls. In this study, cold stress (4°C) and ABA treatment did not affect CMO and BADH expression in A. tricolor.
The CMO gene in A. tricolor genome is inducible by several types stress factors. Extensive studies on its promoter would enable better understanding of the molecular mechanism of GB synthesis gene function. Characterizing the promoter will be possible for the efficient genetic engineering of stress tolerance in plants.
Abbreviations
- BADH:
-
betaine aldehyde dehydrogenase
- CMO:
-
choline monooxygenase
- GB:
-
glycine betaine
- ABA:
-
abscisic acid
- PMSF:
-
phenylmethyl sulfonylfluoride
- NEM:
-
N-ethylmaleimide
- PAGE:
-
polyacrylamide gel electrophoresis
- bp:
-
base pair
- kb:
-
kilobase
- kDa:
-
kilodalton
References
Rhodes D, Hanson AD . Quaternary ammonium and tertiary sulfonium compounds in higher plants. Ann Rev Plant Physiol Plant Mol Biol 1993; 44:357–84.
Valenzuela-Soto E.M . and Mun"oz-Clares, R.A. . Purification and properties of betaine aldehyde dehydrogenase extracted from detached leaves of Amaranthus hypochonodriacus L. subjected to water deficit. J Plant Physiol 1994; 143:145–52.
Weretilnyk EA, Bednarek S, McCue KF, Rhodes D, Hanson AD . Comparative biochemical and immunological studies of the glycine betaine synthesis pathway in diverse families of dicotyledons. Planta 1989; 178:342–52.
Russell B.L, Rathinasabapathi B . and Hanson A . D. Osmotic stress induces expression of choline monooxygenase in sugar beet and amaranth. Plant Physiol 1998; 116:859–65.
Wang Y, Meng YL, Ishikawa H et al. Photosynthetic adaptation to salt stress in three-color leaves of a C4 plant Amranthus tricolor. Plant Cell Physiol 1999; 40:668–74.
Rathinasabapathi B, Burnet M, Russell BL et al. Choline monooxygenase, an unusual iron-sulfur enzyme catalyzing the first step of glycine betaine synthesis in plant: prosthetic group characterization and cDNA cloning. Proc Natl Acad Sci USA 1997; 94:3454–8.
Brouquisse R, Weigel P, Rhodes D, Yocum CF Hanson AD . Evidence for a ferredoxin-dependent choline monooxygenase from spinach chloroplast stroma. Plant Physiol. 1989; 90:322–9.
Burnet M, Lafontaine PJ, Hanson AD . Assay, purification, and partial characterization of choline monooxygenase from spinach. Plant Physiol. 1995; 108:581–8.
Legaria J, Rajsbaum R, Mu\"noz-Clares RA, Villegas-Sepulveda N, Simpson J, Iturriaga G . Molecular characterization of two genes encoding betaine aldehyde dehydrogenase from amaranth. Expression in leaves under short-term exposure to osmotic stress or abscisic acid. Gene 1998; 218:69–76.
Weretilnyk EA, Hanson AD . Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci USA 1990; 87:2745–9.
McCue KF, Hanson AD . Salt-inducible betaine aldehyde dehydrogenase from sugar beet: cDNA cloning and expression. Plant Mol Biol 1992; 18:1–11.
Ishitani M, Nakamura T, Han SY, Takabe T . Expression of the betaine aldehyde dehydrogenase gene in barley response to osmotice stress and abscisic acid. Plant Mol Biol 1995; 27:307–15.
Wood AJ, Saneoka H, Rhodes D, Joly RJ, Goldsbrough PB . Betaine aldehyde dehydrogenase in sorghum. Plant Physiol 1996; 110:1301–8.
Nakamura T, Yokota S, Muramoto Y et al. Expression of a betaine aldehyde dehydrogenase gene in rice, a glycinebetaine nonaccumulator, and possible localization of its protein in peroxisomes. Plant J 1997; 11:1115–20.
Yamamoto N, Mukai Y, Matsuoka M et al. Light-independent expression of cab and rbcS genes in dark-grown pine seedlings. Plant Physiol 1991; 95:379–83.
Alfandari D, Darribere T . A simple PCR method for screening cDNA libraries. PCR Methods Application 1994; 4:46–9.
Chen XY, Chen Y, Heinstein P, Davisson JV . Cloning, expression, and characterization of (+)-d-cadinene synthase: a catalyst for cotton phytoalexin biosynthesis. Arch. Biochem Biophys 1995; 324:255–66.
Futterer J, Gisel A, Iglesias V et al. Standard molecular techniques for the analysis of transgenic plants. In: Potrykus I. and Spangenberg G. (eds)Gene transfer to plants. Springer, Berlin 1995; pp.215–63.
Cline K, Henry R . Import and routing of nucleus-encoded chloroplast proteins. Ann Rev Cell Develop Biol 1996; 12:1–6.
Jiang H, Parales RE, Lynch NA, Gibson DT . Site-directed mutagenesis of conserved amino acids in the alpha subunit of toluene dioxygenase: potental monouclear non-heme iron coordination sites. J Bacteriol 1996; 178:3133–9.
Gray J, Close PS, Briggs SP, Johal GS . A novel suppressor of cell death in plant encoded by the L1s1 gene of maize. Cell 1997; 89:25–31.
Trossat C, Rathinasabapathi B, Hanson AD . Transgenically expressed betaine aldehyde dehydrogenase efficiently catalyzes oxidation of dimethylsulfoniopropionaldehyde and w-aminoaldehydes. Plant physiol. 1997; 113:1457–61.
Vojtê chova M, Hanson A.D . and Mu∼noz-Clares, R.A . Betaine-aldehyde dehydrogenase from amaranth leaves efficiently catalyzes the NAD-dependent oxidation to dimethylsulfoniopropionaldehydeto dimethylsulfoniopropionate. Arch Biochem Biophys 1997; 337:81–8.
Ingram J . and Bartels D . The molecular basis of dehydration tolerance in plants. Ann Rev Plant Physiol Plant Mol Biol 1996; 47:377–403.
Zeevaart JAD, Creelman RA . Metabolism and physiology of abscisic acid. Ann Rev Plant Physiol Plant Mol Biol 1988; 39:439–73.
Kishitani S, Watanabe S, Yasuda S, Arakawa K, Takabe T . Accumulation of glycinebetaine during cold acclimation and freezing tolerance in leaves of winter and spring barley plants. Plant Cell Environ 1994; 17:89–95.
Naidu BP, Paleg LG, Aspinall D, Jennings AC, Jones GP . Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry 1991; 30:407–9.
Acknowledgements
We thank Dr. X.Y. Chen (Shanghai Institute of Plant Physiology, Academia Sinica) for his support of the work, and also members of his Lab. for help and discussion.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
MENG, Y., WANG, Y., ZHANG, D. et al. Isolation of a choline monooxygenase cDNA clone from Amaranthus tricolor and its expressions under stress conditions. Cell Res 11, 187–193 (2001). https://doi.org/10.1038/sj.cr.7290085
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290085
Keywords
This article is cited by
-
The SlCMO Gene Driven by Its Own Promoter Enhances Salt Tolerance of Transgenic Tomato Without Affecting Growth and Yield
Plant Molecular Biology Reporter (2018)
-
Thermophilic bacteria are potential sources of novel Rieske non-heme iron oxygenases
AMB Express (2017)
-
Proteome-level investigation of Cucumis sativus-derived resistance to Sphaerotheca fuliginea
Acta Physiologiae Plantarum (2014)
-
Salt- and osmotic stress-induced choline monooxygenase expression in Kochia scoparia is ABA-independent
Biologia plantarum (2012)
-
Osmoregulation mechanism of drought stress and genetic engineering strategies for improving drought resistance in plants
Forestry Studies in China (2004)