Summary:
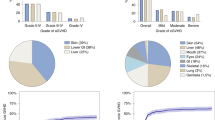
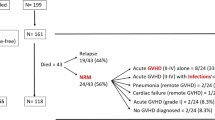
It is unknown whether imatinib prior to myeloablative haematopoietic stem cell transplantation (HSCT) increases transplant-related toxicity. Among the side effects induced by imatinib, myelosuppression and liver injury might worsen HSCT outcomes. We retrospectively analysed engraftment, liver toxicity, acute graft-versus-host disease (aGVHD) incidence and 100-day mortality in 30 patients with BCR/ABL-positive leukaemias who received imatinib before HSCT and compared results of 48 age-matched controls who did not receive preceding imatinib. Both neutrophil and platelet engraftment occurred more rapidly among imatinib patients but the differences adjusted for Gratwohl scale were not statistically significant (P=0.18 and 0.22, respectively). The adjusted hazards of having liver function tests (LFTs) >1.5 normal increased and the adjusted durations of elevated LFTs were not significantly different. The estimated adjusted difference in mean peak bilirubin values was also not significantly different (P=0.48). However, the adjusted hazard of increased creatinine >1.5 normal was significantly higher in the imatinib group (HR=4.09, P=0.02). The adjusted odds of grades II–IV aGVHD were similar in both groups (OR=0.86, P=0.78), and while the adjusted odds of 100-day mortality were lower among imatinib patients, the difference was not significant (OR=0.65, P=0.60). These data do not provide any evidence that imatinib preceding HSCT increases acute transplant-related toxicities.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Silver RT, Woolf SH, Hehlmann R et al. An evidence-based analysis of the effect of busulfan, hydroxyurea, interferon, and allogeneic bone marrow transplantation in treating the chronic phase of chronic myeloid leukemia: developed for the American Society of Hematology. Blood 1999; 94: 1517–1536.
Gale RP, Hehlmann R, Zhang MJ et al. Survival with bone marrow transplantation versus hydroxyurea or interferon for chronic myelogenous leukemia. The German CML Study Group. Blood 1998; 91: 1810–1819.
Druker BJ, Talpaz M, Resta DJ et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med 2001; 344: 1031–1037.
O'Brien SG, Guilhot F, Larson RA et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med 2003; 348: 994–1004.
Gratwohl A, Baldomero H, Passweg J, Urbano-Ispizua A . Increasing use of reduced intensity conditioning transplants: report of the 2001 EBMT activity survey. Bone Marrow Transplant 2002; 30: 813–831.
Giralt S, Sobocinski K, Horowitz MM . Impact of imatinib therapy on the use of allogeneic hematopoietic stem cell transplantation (HSCT) for Treatment of the chronic myelogenous leukemia (CML). Blood 2003; 102: 473a (abstract 1725).
Hochhaus A, Kreil S, Corbin AS et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia 2002; 16: 2190–2196.
Branford S, Rudzki Z, Walsh S et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood 2003; 102: 276–283.
Wassmann B, Pfeifer H, Scheuring UJ et al. Early prediction of response in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ALL) treated with imatinib. Blood 2004; 103: 1495–1498.
Lee S, Kim DW, Kim YJ et al. Minimal residual disease-based role of imatinib as a first-line interim therapy prior to allogeneic stem cell transplantation in Philadelphia chromosome-positive acute lymphoblastic leukemia. Blood 2003; 102: 3068–3070.
Wassmann B, Pfeifer H, Scheuring U et al. Therapy with imatinib mesylate (Glivec) preceding allogeneic stem cell transplantation (SCT) in relapsed or refractory Philadelphia-positive acute lymphoblastic leukemia (Ph+ALL). Leukemia 2002; 16: 2358–2365.
Gupta V, Kamel-Reid S, Minden MD et al. Imatinib mesylate (Gleevec) is a useful agent in the salvage treatment of adults with relapsed/refractory Philadelphia positive acute leukemias. Hematology 2003; 8: 139–143.
Hensley ML, Ford JM . Imatinib treatment: specific issues related to safety, fertility, and pregnancy. Semin Hematol 2003; 40 (2 Suppl. 2): 21–25.
Deininger MW, O'Brien SG, Ford JM, Druker BJ . Practical management of patients with chronic myeloid leukemia receiving imatinib. J Clin Oncol 2003; 21: 1637–1647.
Strasser S, McDonald G . Hepatobiliary complications of hematopoietic cell transplantation. In: Schiff E, Sorrell M, Maddrey W (eds). Schiff's Diseases of the Liver. J.B. Lippincott Company: Philadelphia, PA, 2003, pp 1636–1663.
Gratwohl A, Hermans J, Goldman JM et al. Risk assessment for patients with chronic myeloid leukaemia before allogeneic blood or marrow transplantation. Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Lancet 1998; 352: 1087–1092.
Tutschka PJ, Copelan EA, Klein JP . Bone marrow transplantation for leukemia following a new busulfan and cyclophosphamide regimen. Blood 1987; 70: 1382–1388.
Casper J, Knauf W, Kiefer T et al. Treosulfan and fludarabine: a new toxicity-reduced conditioning regimen for allogeneic hematopoietic stem cell transplantation. Blood 2004; 103: 725–731.
Storb R, Deeg HJ, Whitehead J et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 1986; 314: 729–735.
Przepiorka D, Weisdorf D, Martin P et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
McDonald GB, Hinds MS, Fisher LD et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med 1993; 118: 255–267.
McDonald G, Schoch H, Gooley T . Liver dysfunction and mortality after allogeneic marrow transplantation: analysis of 1419 consecutive patients. Hepatology 1999; 30: 162A (abstract).
Zander AR, Zabelina T, Renges H et al. Pretreatment with Glivec increases transplant-related mortality after allogeneic transplant. Blood 2003; 102: 468a (abstract 1708).
Shimoni A, Kroger N, Zander AR et al. Imatinib mesylate (STI571) in preparation for allogeneic hematopoietic stem cell transplantation and donor lymphocyte infusions in patients with Philadelphia-positive acute leukemias. Leukemia 2003; 17: 290–297.
Oehler V, Gooley T, Snyder D et al. A retrospective study of patients treated with imatinib mesylate prior to allogeneic hematopoietic stem cell transplant. Blood 2004; 104: 753a (abstract 2752).
Ottmann OG, Druker BJ, Sawyers CL et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood 2002; 100: 1965–1971.
van Oosterom AT, Judson I, Verweij J et al. Safety and efficacy of imatinib (STI571) in metastatic gastrointestinal stromal tumours: a phase I study. Lancet 2001; 358: 1421–1423.
Le Coutre P, Pursche S, Baskaynak G et al. Pharmacokinetics and cellular uptake of imatinib and its main metabolite N-desmethyl-imatinib. Blood 2003; 102: 206b (abstract 4545).
Savage DG, Antman KH . Imatinib mesylate – a new oral targeted therapy. N Engl J Med 2002; 346: 683–693.
Hoepfl J, Miething C, Grundler R et al. Effects of imatinib on bone marrow engraftment in syngeneic mice. Leukemia 2002; 16: 1584–1588.
Deininger MW, Goldman JM, Lydon N, Melo JV . The tyrosine kinase inhibitor CGP57148B selectively inhibits the growth of BCR–ABL-positive cells. Blood 1997; 90: 3691–3698.
Prejzner W, Knopinska-Posluszny W, Mikolajczuk J et al. Modification of the clonogenic capacity of bone marrow cells from normal individuals by the tyrosine kinase inhibitor STI571. Clin Lab Haematol 2003; 25: 293–296.
Koh LP, Hwang WY, Chuah CT et al. Imatinib mesylate (STI-571) given concurrently with nonmyeloablative stem cell transplantation did not compromise engraftment and resulted in cytogenetic remission in a patient with chronic myeloid leukemia in blast crisis. Bone Marrow Transplant 2003; 31: 305–308.
Wadhwa J, Szydlo RM, Apperley JF et al. Factors affecting duration of survival after onset of blastic transformation of chronic myeloid leukemia. Blood 2002; 99: 2304–2309.
Sohn SK, Kim JG, Kim DH, Lee KB . Cardiac morbidity in advanced chronic myelogenous leukaemia patients treated by successive allogeneic stem cell transplantation with busulphan/cyclophosphamide conditioning after imatinib mesylate administration. Br J Haematol 2003; 121: 469–472.
Radich JP, Olavarria E, Apperley JF . Allogeneic hematopoietic stem cell transplantation for chronic myeloid leukemia. Hematol Oncol Clin North Am 2004; 18: 685–702.
Acknowledgements
We appreciate the work of the staff that provided the excellent care to all patients.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaucha, J., Prejzner, W., Giebel, S. et al. Imatinib therapy prior to myeloablative allogeneic stem cell transplantation. Bone Marrow Transplant 36, 417–424 (2005). https://doi.org/10.1038/sj.bmt.1705087
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705087
Keywords
This article is cited by
-
The prevalence of hepatic and thyroid toxicity associated with imatinib treatment of chronic myeloid leukaemia: a systematic review
International Journal of Clinical Pharmacy (2023)
-
Outcomes and toxicity of allogeneic hematopoietic cell transplantation in chronic myeloid leukemia patients previously treated with second-generation tyrosine kinase inhibitors: a prospective non-interventional study from the Chronic Malignancy Working Party of the EBMT
Bone Marrow Transplantation (2022)
-
Allogeneic transplantation for CML in the TKI era: striking the right balance
Nature Reviews Clinical Oncology (2016)
-
Pretransplantation use of the second-generation tyrosine kinase inhibitors has no negative impact on the HCT outcome
Annals of Hematology (2015)
-
Prognostic factors for outcomes in allogeneic transplantation for CML in the imatinib era: a CIBMTR analysis
Bone Marrow Transplantation (2012)