Key Points
-
The recent DNA sequence determination of the human, mouse and rice genomes has highlighted the abundant and diverse nature of transposons; approximately 40% of the genomes are composed of repetitive elements.
-
The mechanisms for how these elements move and cause chromosomal rearrangements are derived from the many elegant genetic, molecular and structural studies carried out on transposons found in model organisms.
-
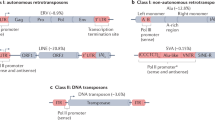
Transposons can be organized into five families based on their encoded transposases, which dictate how they move. Although mechanistic details of transposition have been determined for a relatively small number of elements, the organization of transposons based on their probable catalytic mechanism provides a useful framework for predicting the movement and rearrangements caused by the elements still to be characterized.
-
DDE-transposons encode a transposase that has a related amino-acid motif (the DDE motif), which forms the active site of the transposase and is responsible for coordinating the cleavage and joining steps of transposition.
-
Tyrosine (Y)-transposons encode transposases that are related to tyrosine recombinases, which normally promote site-specific recombination. These elements transpose using an active-site tyrosine to cleave transposon and target DNA. Importantly, theY-transposases lack the site-specificity of their site-specific cousins and therefore have the ability to insert into many different target sites.
-
Serine (S)-transposons, which are newly discovered elements, encode transposases that are related to site-specific serine recombinases. As for Y-transposases, it is thought that the ability of S-transposases to recognize different sequences allows S-transposons to insert into unrelated targets.
-
Y2-transposons (or rolling-circle transposons) move by a mechanism that resembles rolling-circle replication as carried out by many bacterial plasmids and phage. The Y2-transposase contains several motifs (including a pair of tyrosine residues) that are conserved among plasmid and phage rolling-circle replication proteins, which are important for DNA binding and catalysis.
-
Target-primed (TP)-retrotransposons use a combination of reverse transcriptase and endonuclease activities to reverse transcribe an RNA copy of their genome directly into a target.
Abstract
Transposons are ubiquitous in prokaryotic and eukaryotic organisms and are major determinants of genome structure. Transposition — the movement of discrete segments of DNA without a requirement for homology — occurs by a handful of mechanisms that are used over and over again in different combinations. Understanding these mechanisms provides an important key to unlocking the secrets of genome organization and evolution.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Watson, J. D. & Crick, F. H. A structure for deoxyribose nucleic acid. Nature 171, 737–738 (1953).
Lander, E. S. et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Waterston, R. H. et al. Initial sequencing and comparative analysis of the mouse genome. Nature 420, 520–562 (2002).
Goff, S. A. et al. A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296, 92–100 (2002).
Yu, J. et al. A draft sequence of the rice genome (Oryza sativa L. ssp. indica). Science 296, 79–92 (2002).
Berg, D. E. & Howe, M. M. Mobile DNA (American Society for Microbiology, Washington DC, 1989).
Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M. Mobile DNA II, (American Society for Microbiology, Washington DC, 2002). This compilation of reviews and its parent (reference 6) are the 'bibles' of transposition. We refer interested readers to these books for more detailed descriptions of the many different types of mobile element.
Moran, J. V., DeBerardinis, R. J. & Kazazian, H. H. Jr. Exon shuffling by L1 retrotransposition. Science 283, 1530–1534 (1999).
Mendiola, M. V., Bernales, I. & de la Cruz, F. Differential roles of the transposon termini in IS91 transposition. Proc. Natl Acad. Sci. USA 91, 1922–1926 (1994). A model for Y2-transposition is presented based on the similarity of these elements and their transposases to rolling-circle replication systems that are found in certain bacterial plasmids and phage (see reference 79 for an alternative model).
Agrawal, A., Eastman, Q. M. & Schatz, D. G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature 394, 744–751 (1998).
Hiom, K., Melek, M. & Gellert, M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell 94, 463–470 (1998). References 10 and 11 describe in vitro transposition of signal sequences that are mediated by the V( D )J recombination proteins and provide evidence for the evolution of the immune system from a transposon.
Sharp, P. A. “Five easy pieces”. Science 254, 663 (1991).
Eickbush, T. H. Telomerase and retrotransposons: which came first? Science 277, 911–912 (1997).
Nakamura, T. M. & Cech, T. R. Reversing time: origin of telomerase. Cell 92, 587–590 (1998).
McClintock, B. Mutable loci in maize. Carnegie Institute Washington Year Book 47, 155–169 (1948).
Kulkosky, J., Jones, K. S., Katz, R. A., Mack, J. P. & Skalka, A. M. Residues critical for retroviral integrative recombination in a region that is highly conserved among retroviral/retrotransposon integrases and bacterial insertion sequence transposases. Mol. Cell. Biol. 12, 2331–2338 (1992).
Haren, L., Ton-Hoang, B. & Chandler, M. Integrating DNA: transposases and retroviral integrases. Annu. Rev. Microbiol. 53, 245–281 (1999).
Van Duyne, G. D. in Mobile DNA II Vol. II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 93–117 (American Society for Microbiology, Washington DC, 2002).
Azaro, M. A. & Landy, A. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 118–148 (American Society for Microbiology Press, Washington DC, 2002).
Grindley, N. D. F in Mobile DNA II Vol. II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 272–302 (American Society for Microbiology, Washington DC, 2002).
Churchward, G. in Mobile DNA II Vol. II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 177–191 (American Society for Microbiology, Washington DC, 2002).
Burrus, V., Pavlovic, G., Decaris, B. & Guedon, G. Conjugative transposons: the tip of the iceberg. Mol. Microbiol. 46, 601–610 (2002).
Goodwin, T. J. & Poulter, R. T. The DIRS1 group of retrotransposons. Mol. Biol. Evol. 18, 2067–2082 (2001).
Duncan, L., Bouckaert, K., Yeh, F. & Kirk, D. L. Kangaroo, a mobile element from Volvox carteri, is a member of a newly recognized third class of retrotransposons. Genetics 162, 1617–1630 (2002).
Garcillan-Barcia, M. P., Bernales, I., Mendiola, M. V. & de la Cruz, F. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 891–904 (American Society for Microbiology, Washington DC, 2002).
Kapitonov, V. V. & Jurka, J. Rolling-circle transposons in eukaryotes. Proc. Natl Acad. Sci. USA 98, 8714–8719 (2001). The discovery of helitrons by a computational approach is reported, and a model for their mechanism of mobility is presented based on reference 9.
Eickbush, T. H. & Malik, H. S. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 1111–1144 (American Society for Microbiology, Washington DC, 2002).
Craigie, R. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 613–630 (American Society for Microbiology, Washington DC, 2002).
Cappello, J., Handelsman, K. & Lodish, H. F. Sequence of Dictyostelium DIRS-1: an apparent retrotransposon with inverted terminal repeats and an internal circle junction sequence. Cell 43, 105–115 (1985). The initial description of the DIRS1 transposon of Dictyostelium discoideum as a retrotransposon and a model for its mechanism of reverse transcription and transposition through a circular cDNA intermediate are presented.
Luan, D. D., Korman, M. H., Jakubczak, J. L. & Eickbush, T. H. Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell 72, 595–605 (1993). The mechanism of TP reverse transcription was determined by in vitro analysis of the endonuclease and reverse transcriptase activities of the RT/En protein encoded by the R2Bm element of Bombyx mori.
Cost, G. J., Feng, Q., Jacquier, A. & Boeke, J. D. Human L1 element target-primed reverse transcription in vitro. EMBO J. 21, 5899–5910 (2002).
Chandler, M. & Mahillon, J. in Mobile DNA II Vol. II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 305–366 (American Society for Microbiology, Washington DC, 2002).
Rezsohazy, R., Hallet, B., Delcour, J. & Mahillon, J. The IS4 family of insertion sequences: evidence for a conserved transposase motif. Mol. Microbiol. 9, 1283–1295 (1993).
Rice, P. & Mizuuchi, K. Structure of the bacteriophage Mu transposase core: a common structural motif for DNA transposition and retroviral integration. Cell 82, 209–220 (1995). References 34–40 describe and compare the structures of several DDE-transposases and integrases, highlighting their structural similarity despite their sequence diversity.
Davies, D. R., Goryshin, I. Y., Reznikoff, W. S. & Rayment, I. The three-dimensional structure of the Tn5 synaptic complex intermediate. Science 289, 77–85 (2000). This study presents the first structure of a synaptic complex between the transposase and excised transposon ends.
Bujacz, G. et al. High-resolution structure of the catalytic domain of avian sarcoma virus integrase. J. Mol. Biol. 253, 333–346 (1995).
Bujacz, G., Alexandratos, J., Qing, Z. L., Clement-Mella, C. & Wlodawer, A. The catalytic domain of human immunodeficiency virus integrase: ordered active site in the F185H mutant. FEBS Lett. 398, 175–178 (1996).
Dyda, F. et al. Crystal structure of the catalytic domain of HIV-1 integrase: similarity to other polynucleotidyl transferases. Science 266, 1981–1986 (1994).
Bujacz, G. et al. Binding of different divalent cations to the active site of avian sarcoma virus integrase and their effects on enzymatic activity. J. Biol. Chem. 272, 18161–18168 (1997).
Lovell, S., Goryshin, I. Y., Reznikoff, W. R. & Rayment, I. Two-metal active-site binding of a Tn5 transposase synaptic complex. Nature Struct. Biol. 9, 278–281 (2002).
Mizuuchi, K. & Baker, T. A. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 12–23 (American Society for Microbiology, Washington DC, 2002).
Mizuuchi, M., Baker, T. A. & Mizuuchi, K. Assembly of phage Mu transpososomes: cooperative transitions assisted by protein and DNA scaffolds. Cell 83, 375–385 (1995).
Surette, M. G., Buch, S. J. & Chaconas, G. Transpososomes: stable protein–DNA complexes involved in the in vitro transposition of bacteriophage Mu DNA. Cell 49, 253–262 (1987).
Turlan, C. & Chandler, M. Playing second fiddle: second-strand processing and liberation of transposable elements from donor DNA. Trends Microbiol. 8, 268–274 (2000).
Craigie, R. & Mizuuchi, K. Mechanism of transposition of bacteriophage Mu: structure of a transposition intermediate. Cell 41, 867–876 (1985).
Mizuuchi, K. Mechanism of transposition of bacteriophage Mu: polarity of the strand transfer reaction at the initiation of transposition. Cell 39, 395–404 (1984). This classic paper and other papers by Mizuuchi and colleagues describe the establishment of the first in vitro transposition system. Importantly, they assign the polarity of strand transfer, identify and characterize the strand-transfer intermediate and define the chemistry of the reaction.
Shapiro, J. A. Molecular model for the transposition and replication of bacteriophage Mu and other transposable elements. Proc. Natl Acad. Sci. USA 76, 1933–1937 (1979).
Bhasin, A., Goryshin, I. Y. & Reznikoff, W. S. Hairpin formation in Tn5 transposition. J. Biol. Chem. 274, 37021–37029 (1999).
Kennedy, A. K., Guhathakurta, A., Kleckner, N. & Haniford, D. B. Tn10 transposition via a DNA hairpin intermediate. Cell 95, 125–134 (1998).The first demonstration of the involvement of hairpins in the excision of transposons from their flanking donor DNA (see also reference 48). The mechanism explains how a single-protein active site can cleave multiple DNA strands by using reiterative steps of hydrolysis and trans -esterification.
Engels, W. R., Johnson-Schlitz, D. M., Eggleston, W. B. & Sved, J. High-frequency P element loss in Drosophila is homolog dependent. Cell 62, 515–525 (1990).
Plasterk, R. H. & Groenen, J. T. Targeted alterations of the Caenorhabditis elegans genome by transgene instructed DNA double strand break repair following Tc1 excision. EMBO J. 11, 287–290 (1992).
Coen, E. S., Carpenter, R. & Martin, C. Transposable elements generate novel spatial patterns of gene expression in Antirrhinum majus. Cell 47, 285–296 (1986).
Kunze, R. & Weil, C. F. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 565–610 (American Society for Microbiology, Washington DC, 2002).
Roth, D. B. & Craig, N. L. VDJ recombination: a transposase goes to work. Cell 94, 411–414 (1998).
Gellert, M. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 705–729 (American Society for Microbiology, Washington DC, 2002).
Dawson, A. & Finnegan, D. J. Excision of the Drosophila mariner transposon Mos1. Comparison with bacterial transposition and V(D)J recombination. Mol. Cell. 11, 225–235 (2003).
Biery, M. C., Stewart, F. J., Stellwagen, A. E., Raleigh, E. A. & Craig, N. L. A simple in vitro Tn7-based transposition system with low target site selectivity for genome and gene analysis. Nucl. Acids Res. 28, 1067–1077 (2000).
Sarnovsky, R. J., May, E. W. & Craig, N. L. The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. EMBO J. 15, 6348–6361 (1996).
Waddell, C. S. & Craig, N. L. Tn7 transposition: two transposition pathways directed by five Tn7-encoded genes. Genes Dev. 2, 137–149 (1988).
Craig, N. L. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 423–456 (American Society for Microbiology, Washington DC, 2002).
May, E. W. & Craig, N. L. Switching from cut-and-paste to replicative Tn7 transposition. Science 272, 401–404 (1996).
Hickman, A. B. et al. Unexpected structural diversity in DNA recombination: the restriction endonuclease connection. Mol. Cell 5, 1025–1034 (2000).
Rousseau, P. et al. in Mobile DNA II Vol. II (eds. Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 367–383 (American Society for Microbiology, Washington DC, 2002).
Sekine, Y., Aihara, K. & Ohtsubo, E. Linearization and transposition of circular molecules of insertion sequence IS3. J. Mol. Biol. 294, 21–34 (1999).
Lewis, L. A. & Grindley, N. D. Two abundant intramolecular transposition products, resulting from reactions initiated at a single end, suggest that IS2 transposes by an unconventional pathway. Mol. Microbiol. 25, 517–529 (1997).
Polard, P. & Chandler, M. An in vivo transposase-catalyzed single-stranded DNA circularization reaction. Genes Dev. 9, 2846–2858 (1995).
Ton-Hoang, B., Polard, P., Haren, L., Turlan, C. & Chandler, M. IS911 transposon circles give rise to linear forms that can undergo integration in vitro. Mol. Microbiol. 32, 617–627 (1999).
Boeke, J. D., Garfinkel, D. J., Styles, C. A. & Fink, G. R. Ty elements transpose through an RNA intermediate. Cell 40, 491–500 (1985).
Garfinkel, D. J., Boeke, J. D. & Fink, G. R. Ty element transposition: reverse transcriptase and virus-like particles. Cell 42, 507–517 (1985). References 68 and 69 represent the first demonstration that a retrotransposon moves through an RNA intermediate. Moreover, reverse transcription of Ty1 RNA was shown to occur in cytoplasmic virus-like particles.
Fujiwara, T. & Mizuuchi, K. Retroviral DNA integration: structure of an integration intermediate. Cell 54, 497–504 (1988).
Kirchner, J. & Sandmeyer, S. B. Ty3 integrase mutants defective in reverse transcription or 3′-end processing of extrachromosomal Ty3 DNA. J. Virol. 70, 4737–4747 (1996).
Eichinger, D. J. & Boeke, J. D. A specific terminal structure is required for Ty1 transposition. Genes Dev. 4, 324–330 (1990).
Kahn, S. A. Plasmid rolling-circle replication: recent developments. Mol. Microbiol. 37, 477–484 (2000).
Novick, R. P. Contrasting lifestyles of rolling-circle phages and plasmids. Trends Biochem. Sci. 23, 434–438 (1998).
Hickman, A. B., Ronning, D. R., Kotin, R. M. & Dyda, F. Structural unity among viral origin binding proteins: crystal structure of the nuclease domain of adeno-associated virus Rep. Mol. Cell 10, 327–337 (2002).
Smith, R. H. & Kotin, R. M. An adeno-associated virus (AAV) initiator protein, Rep78, catalyzes the cleavage and ligation of single-stranded AAV ori DNA. J. Virol. 74, 3122–3129 (2000).
Smith, R. H. & Kotin, R. M. in Mobile DNA II, Vol. II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 905–926 (American Society for Microbiology, Washington DC, 2002).
Garcillan-Barcia, M. P., Bernales, I., Mendiola, M. V. & de la Cruz, F. Single-stranded DNA intermediates in IS91 rolling-circle transposition. Mol. Microbiol. 39, 494–501 (2001).
Tavakoli, N. et al. IS1294, a DNA element that transposes by RC transposition. Plasmid 44, 66–84 (2000).
Kapitonov, V. V. & Jurka, J. Molecular paleontology of transposable elements in the Drosophila melanogaster genome. Proc. Natl Acad. Sci. USA 100, 6569–6574 (2003).
Lal, S. K., Giroux, M. J., Brendel, V., Vallejos, C. E. & Hannah, L. C. The maize genome contains a helitron insertion. Plant Cell 15, 381–391 (2003).
Louis, E. J. & Haber, J. E. The structure and evolution of subtelomeric Y′ repeats in Saccharomyces cerevisiae. Genetics 131, 559–574 (1992).
Horowitz, H. & Haber, J. E. Identification of autonomously replicating circular subtelomeric Y′ elements in Saccharomyces cerevisiae. Mol. Cell Biol. 5, 2369–2380 (1985).
Nash, H. A. in Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology 2nd edn (eds Neidhardt, F. C. et al.) 2363–2376 (American Society for Microbiology, Washington DC, 1996).
Wang, H. et al. Characterization of the ends and target sites of the novel conjugative transposon Tn5397 from Clostridium difficile: excision and circularization is mediated by the large resolvase, TndX. J. Bacteriol. 182, 3775–3783 (2000).
Hochhut, B. & Waldor, M. K. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC. Mol. Microbiol. 32, 99–110 (1999).
Kersulyte, D., Mukhopadhyay, A. K., Shirai, M., Nakazawa, T. & Berg, D. E. Functional organization and insertion specificity of IS607, a chimeric element of Helicobacter pylori. J. Bacteriol. 182, 5300–5308 (2000).
Rudy, C., Taylor, K. L., Hinerfeld, D., Scott, J. R. & Churchward, G. Excision of a conjugative transposon in vitro by the Int and Xis proteins of Tn916. Nucl. Acids Res. 25, 4061–4066 (1997).
Kitts, P. A. & Nash, H. A. Homology-dependent interactions in phage λ site-specific recombination. Nature 329, 346–348 (1987).
Nunes-Duby, S. E., Yu, D. & Landy, A. Sensing homology at the strand-swapping step in λ excisive recombination. J. Mol. Biol. 272, 493–508 (1997).
Caparon, M. G. & Scott, J. R. Excision and insertion of the conjugative transposon Tn916 involves a novel recombination mechanism. Cell 59, 1027–1034 (1989).
Trieu-Cuot, P., Poyart-Salmeron, C., Carlier, C. & Courvalin, P. Sequence requirements for target activity in site-specific recombination mediated by the Int protein of transposon Tn1545. Mol. Microbiol. 8, 179–185 (1993).
Jacobs, M. E., Sanchez-Blanco, A., Katz, L. A. & Klobutcher, L. A. Tec3, a new developmentally eliminated dna element in Euplotes crassus. Eukaryot. Cell 2, 103–114 (2003).
Doak, T. G., Witherspoon, D. J., Jahn, C. L. & Herrick, G. Selection on the genes of Euplotes crassus Tec1 and Tec2 transposons: evolutionary appearance of a programmed frameshift in a Tec2 gene encoding a tyrosine family site-specific recombinase. Eukaryot. Cell 2, 95–102 (2003).
Klobutcher, L. A., Turner, L. R. & LaPlante, J. Circular forms of developmentally excised DNA in Euplotes crassus have a heteroduplex junction. Genes Dev. 7, 84–94 (1993).
Crellin, P. K. & Rood, J. I. The resolvase/invertase domain of the site-specific recombinase TnpX is functional and recognizes a target sequence that resembles the junction of the circular form of the Clostridium perfringens transposon Tn4451. J. Bacteriol. 179, 5148–5156 (1997).
Wang, H. & Mullany, P. The large resolvase TndX is required and sufficient for integration and excision of derivatives of the novel conjugative transposon Tn5397. J. Bacteriol. 182, 6577–6583 (2000).
Johnson, R. C. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 230–271 (American Society for Microbiology, Washington DC, 2002).
Bannam, T. L., Crellin, P. K. & Rood, J. I. Molecular genetics of the chloramphenicol-resistance transposon Tn4451 from Clostridium perfringens: the TnpX site-specific recombinase excises a circular transposon molecule. Mol. Microbiol. 16, 535–551 (1995).
Stark, W. M., Grindley, N. D. F., Hatfull, G. F. & Boocock, M. R. Resolvase-catalysed reactions between res sites differing in the central dinucleotide of subsite I. EMBO J. 10, 3541–3548 (1991).
Prak, E. T. & Kazazian, H. H. Jr. Mobile elements and the human genome. Nature Rev. Genet. 1, 134–144 (2000).
Martin, S. L. & Bushman, F. D. Nucleic acid chaperone activity of the ORF1 protein from the mouse LINE-1 retrotransposon. Mol. Cell Biol. 21, 467–475 (2001).
Feng, Q., Moran, J. V., Kazazian, H. H. Jr. & Boeke, J. D. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell 87, 905–916 (1996).
Feng, Q., Schumann, G. & Boeke, J. D. Retrotransposon R1Bm endonuclease cleaves the target sequence. Proc. Natl Acad. Sci. USA 95, 2083–2088 (1998).
Yang, J., Malik, H. S. & Eickbush, T. H. Identification of the endonuclease domain encoded by R2 and other site-specific, non-long terminal repeat retrotransposable elements. Proc. Natl Acad. Sci. USA 96, 7847–7852 (1999).
Arkhipova, I. R., Pyatkov, K. I., Meselson, M. & Evgen'ev, M. B. Retroelements containing introns in diverse invertebrate taxa. Nature Genet. 33, 123–124 (2003).
Morrish, T. A. et al. DNA repair mediated by endonuclease-independent LINE-1 retrotransposition. Nature Genet. 31, 159–165 (2002). Demonstration that the human TP-retrotransposon, LINE-1, can integrate into DNA lesions by an endonuclease-independent mechanism.
Symer, D. E. et al. Human l1 retrotransposition is associated with genetic instability in vivo. Cell 110, 327–338 (2002).
Eickbush, T. H. in Mobile DNA II (eds. Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 813–835 (American Society for Microbiology, Washington DC, 2002).
Belfort, M., Derbyshire, V., Parker, M. M., Cousineau, B. & Lambowitz, A. M. Mobile introns: pathway and proteins. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 761–783 (American Society for Microbiology, Washington DC, 2002).
Cousineau, B., Lawrence, S., Smith, D. & Belfort, M. Retrotransposition of a bacterial group II intron. Nature 404, 1018–1021 (2000).
Cousineau, B. et al. Retrohoming of a bacterial group II intron: mobility via complete reverse splicing, independent of homologous DNA recombination. Cell 94, 451–462 (1998).
Yang, J., Zimmerly, S., Perlman, P. S. & Lambowitz, A. M. Efficient integration of an intron RNA into double-stranded DNA by reverse splicing. Nature 381, 332–335 (1996).
Zimmerly, S. et al. A group II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell 83, 529–538 (1995).
Zimmerly, S., Guo, H., Perlman, P. S. & Lambowitz, A. M. Group II intron mobility occurs by target DNA-primed reverse transcription. Cell 82, 545–554 (1995). References 114 and 115 represent the first demonstration of the mechanism of group II intron mobility by TP reverse transcription of an intron RNA that reverse-spliced into a DNA target.
Ichiyanagi, K. et al. Retrotransposition of the Ll. LtrB group II intron proceeds predominantly via reverse splicing into DNA targets. Mol. Microbiol. 46, 1259–1272 (2002).
Pardue, M. -L. & DeBaryshe, P. G. in Mobile DNA II (eds Craig, N. L., Craigie, R., Gellert, M. & Lambowitz, A. M.) 870–887 (American Society for Microbiology, Washington DC, 2002).
Toor, N. & Zimmerly, S. Identification of a family of group II introns encoding LAGLIDADG ORFs typical of group I introns. RNA 8, 1373–1377 (2002).
Fugmann, S. D., Villey, I. J., Ptaszek, L. M. & Schatz, D. G. Identification of two catalytic residues in RAG1 that define a single active site within the RAG1/RAG2 protein complex. Mol. Cell 5, 97–107 (2000).
Kim, D. R., Dai, Y., Mundy, C. L., Yang, W. & Oettinger, M. A. Mutations of acidic residues in RAG1 define the active site of the V(D)J recombinase. Genes Dev. 13, 3070–3080 (1999).
Landree, M. A., Wibbenmeyer, J. A. & Roth, D. B. Mutational analysis of RAG1 and RAG2 identifies three catalytic amino acids in RAG1 critical for both cleavage steps of V(D)J recombination. Genes Dev. 13, 3059–3069 (1999).
van Gent, D. C. et al. Initiation of V(D)J recombination in a cell-free system. Cell 81, 925–934 (1995).
McBlane, J. F. et al. Cleavage at a V(D)J recombination signal requires only RAG1 and RAG2 proteins and occurs in two steps. Cell 83, 387–395 (1995).
Gellert, M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 71, 101–132 (2002).
Dai, Y. et al. Nonhomologous end joining and V(D)J recombination require an additional factor. Proc. Natl Acad. Sci. USA 100, 2462–2467 (2003).
Messier, T. L., O'Neill, J. P., Hou, S. M., Nicklas, J. A. & Finette, B. A. In vivo transposition mediated by V(D)J recombinase in human T lymphocytes. EMBO J. 22, 1381–1388 (2003).
Hanai, R. & Wang, J. C. The mechanism of sequence-specific DNA cleavage and strand transfer by phi X174 gene A* protein. J. Biol. Chem. 268, 23830–23836 (1993).
van Mansfeld, A. D., van Teeffelen, H. A., Baas, P. D. & Jansz, H. S. Two juxtaposed tyrosyl-OH groups participate in phi X174 gene A protein catalysed cleavage and ligation of DNA. Nucl. Acids Res. 14, 4229–4238 (1986).
Zechner, E. L. et al. in The Horizontal Gene Pool, Bacterial Plasmids and Gene Spread (ed. Thomas, C. M.) 87–175 (Harwood Academic Publishers, Amsterdam, 2000).
Acknowledgements
We thank M. Belfort for comments on this work and, in particular, N. Grindley for his enthusiastic discussions on transposon families and mechanisms. This work was supported by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
Swiss-Prot
FURTHER INFORMATION
Flybase, Drosophila Transposons, Transgene Constructs, and Insertions
Glossary
- TRANSPOSABLE ELEMENT
-
A transposable element or a transposon is a defined segment of DNA that has the ability to move, or copy itself, into a second location without a requirement for DNA homology.
- TRANSPOSASE
-
An enzyme that is responsible for the catalysis of transposition.
- DDE
-
A triad of highly conserved amino acids (aspartate (D), aspartate, glutamate (E)) that is found in one class of transposases, which are required for the coordination of metal ions that are necessary for catalysis.
- REVERSE TRANSCRIPTASE
-
A DNA polymerase that can use RNA as a template for the synthesis of a cDNA.
- ENDONUCLEASE
-
An enzyme that catalyzes the cleavage of a phosphodiester bond within a DNA molecule.
- TYROSINE RECOMBINASE
-
An enzyme that is capable of rearranging DNA using a conserved tyrosine residue to cleave and reseal its substrates by a 3′-phosphotyrosine linkage (also known as integrase).
- SERINE RECOMBINASE
-
Recombination protein (also known as resolvase or invertase) that uses an active-site serine nucleophile to perform strand-cleavage and religation steps through a 5′-phosphoserine intermediate.
- CONJUGATIVE TRANSPOSON
-
A transposon that encodes functions allowing transfer of the transposon DNA between donor and recipient bacterial cells.
- RETROTRANSPOSON
-
A transposon, the movement of which occurs through an RNA intermediate, which is copied into a cDNA molecule.
- LTR
-
(Long terminal repeat). A directly repeated sequence at each end of a retrovirus or retrotransposon, which is necessary for reverse transcription, integration and transcription.
- INTEGRASE
-
A term used to describe two different protein families that integrate one DNA molecule into another, but by two different mechanisms. 1: a DDE-containing protein of LTR-retrotransposons and retroviruses that catalyses the integration of a cDNA molecule into a target DNA. 2: a member of the tyrosine site-specific recombinases that is responsible for catalysing the integration and excision of DNA, most notably bacteriophage λ.
- DNA HYDROLYSIS
-
Cleavage of a DNA backbone by an activated water molecule, resulting in the addition of an OH group to the 3′ or 5′ position of the ribose.
- TRANS-ESTERIFICATION
-
The direct exchange of an alcohol moiety of an ester for another alcohol. In this case, the OH group on the 3′ end of the transposon or an amino acid (Y or S) is exchanged for the phospho-ester group on a DNA target.
- TRANSPOSOSOME
-
A protein–DNA complex that mediates all the steps of transposition, ensuring the fidelity of the reaction. The complex contains both cis-acting sites (for example, transposon ends), target and transposase protein (and sometimes other host factors).
- NUCLEOPHILIC ATTACK
-
A reaction that involves the transfer of electrons from a nucleophile; for example, a hydroxyl group from H2O or a serine or tyrosine residue.
- NON-HOMOLOGOUS END-JOINING
-
The joining of DNA ends that share no, or only a few, nucleotides of DNA homology.
- P NUCLEOTIDE
-
(Palindromic nucleotide). A small, palindromic DNA sequence that is introduced at the site of hairpin resolution during excision of Tam3-related elements and V(D)J signal sequences.
- TYPE IIS RESTRICTION ENDONUCLEASE
-
A DNA endonuclease that recognizes an asymmetric sequence and cleaves both DNA strands at fixed positions outside the recognition site.
- RAG PROTEIN
-
Protein product of the recombination-activating genes that are required for V(D)J recombination.
- SYNAPTIC COMPLEX
-
A protein–DNA complex, or transpososome, that contains DNA sites in the correct alignment for effective recombination.
- RNASE H
-
An enzyme that degrades the RNA strand of an RNA–DNA duplex.
- CONJUGAL RELAXASE
-
A protein that is responsible for initiating DNA transfer in bacteria. It contains a conserved tyrosine motif that is required to nick the transferred DNA strand at oriT, which it forms a 5′-phosphotyrosine bond with.
- HELICASE
-
An enzyme that promotes ATP-dependent disassociation of the complementary DNA or RNA strands of a duplex molecule.
- DEAD BOX
-
An evolutionarily conserved array of amino acids, also known as the Walker B motif, that confers ATP-binding activity.
- LINE
-
(Long interspersed nuclear element). A retrotransposon, the mobility of which is dependent on target-primed reverse transcription.
- GROUP II INTRON
-
An autocatalytic intron from one of two families that catalyses its own splicing from an RNA transcript and encodes a protein that mediates its mobility as a DNA element. Group II introns are the likely progenitors of spliceosomal introns.
Rights and permissions
About this article
Cite this article
Curcio, M., Derbyshire, K. The outs and ins of transposition: from Mu to Kangaroo. Nat Rev Mol Cell Biol 4, 865–877 (2003). https://doi.org/10.1038/nrm1241
Issue Date:
DOI: https://doi.org/10.1038/nrm1241
This article is cited by
-
Host range of strand-biased circularizing integrative elements: a new class of mobile DNA elements nesting in Gammaproteobacteria
Mobile DNA (2023)
-
From parasites to partners: exploring the intricacies of host-transposon dynamics and coevolution
Functional & Integrative Genomics (2023)
-
A unique eukaryotic lineage of composite-like DNA transposons encoding a DDD/E transposase and a His-Me finger homing endonuclease
Mobile DNA (2022)
-
AcademH, a lineage of Academ DNA transposons encoding helicase found in animals and fungi
Mobile DNA (2020)
-
Measuring and interpreting transposable element expression
Nature Reviews Genetics (2020)