Abstract
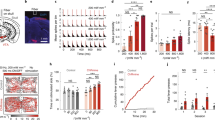
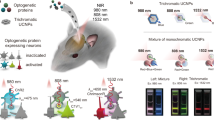
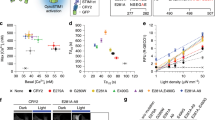
Channelrhodopsins (ChRs) are used to optogenetically depolarize neurons. We engineered a variant of ChR, denoted red-activatable ChR (ReaChR), that is optimally excited with orange to red light (λ ∼590–630 nm) and offers improved membrane trafficking, higher photocurrents and faster kinetics compared to existing red-shifted ChRs. Red light is less scattered by tissue and is absorbed less by blood than the blue to green wavelengths that are required by other ChR variants. We used ReaChR expressed in the vibrissa motor cortex to drive spiking and vibrissa motion in awake mice when excited with red light through intact skull. Precise vibrissa movements were evoked by expressing ReaChR in the facial motor nucleus in the brainstem and illumination with red light through the external auditory canal. Thus, ReaChR enables transcranial optical activation of neurons in deep brain structures without the need to surgically thin the skull, form a transcranial window or implant optical fibers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Arrenberg, A.B., Stainier, D.Y., Baier, H. & Huisken, J. Optogenetic control of cardiac function. Science 330, 971–974 (2010).
Boyden, E.S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Bruegmann, T. et al. Optogenetic control of heart muscle in vitro and in vivo. Nat. Methods 7, 897–900 (2010).
Nagel, G. et al. Channelrhodopsin-1: a light-gated proton channel in green algae. Science 296, 2395–2398 (2002).
Nagel, G. et al. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 100, 13940–13945 (2003).
Adamantidis, A.R., Zhang, F., Aravanis, A.M., Deisseroth, K. & de Lecea, L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature 450, 420–424 (2007).
Lin, J.Y., Lin, M.Z., Steinbach, P. & Tsien, R.Y. Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys. J. 96, 1803–1814 (2009).
Wen, L. et al. Opto-current-clamp actuation of cortical neurons using a strategically designed channelrhodopsin. PLoS ONE 5, e12893 (2010).
Govorunova, E.G., Spudich, E.N., Lane, C.E., Sineshchekov, O.A. & Spudich, J.L. New channelrhodopsin with a red-shifted spectrum and rapid kinetics from Mesostigma viride. MBio. 2, e00115–e001111 (2011).
Kleinlogel, S. et al. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 14, 513–518 (2011).
Yizhar, O. et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477, 171–178 (2011).
Tromberg, B.J. et al. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2, 26–40 (2000).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiberoptic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Zhang, F. et al. Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri. Nat. Neurosci. 11, 631–633 (2008).
Lin, J.Y. A user's guide to channelrhodopsin variants: features, limitations and future developments. Exp. Physiol. 96, 19–25 (2011).
Wang, H. et al. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from Chlamydomonas. J. Biol. Chem. 284, 5685–5696 (2009).
Gunaydin, L.A. et al. Ultrafast optogenetic control. Nat. Neurosci. 13, 387–392 (2010).
Nagel, G. et al. Light activation of channelrhodopsin-2 in excitable cells of Caenorhabditis elegans triggers rapid behavioral responses. Curr. Biol. 15, 2279–2284 (2005).
Griesbeck, O., Baird, G.S., Campbell, R.E., Zacharias, D.A. & Tsien, R.Y. Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications. J. Biol. Chem. 276, 29188–29194 (2001).
Mattis, J. et al. Principles for applying optogenetic tools derived from direct comparative analysis of microbial opsins. Nat. Methods 9, 159–172 (2012).
Hill, D.N., Curtis, J.C., Moore, J.D. & Kleinfeld, D. Primary motor cortex reports efferent control of vibrissa motion on multiple timescales. Neuron 72, 344–356 (2011).
Berg, R.W. & Kleinfeld, D. Vibrissa movement elicited by rhythmic electrical microstimulation to motor cortex in the aroused rat mimics exploratory whisking. J. Neurophysiol. 90, 2950–2963 (2003).
Brecht, M., Schneider, M., Sakmann, B. & Margrie, T.W. Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427, 704–710 (2004).
Haiss, F. & Schwarz, C. Spatial segregation of different modes of movement control in the whisker representation of rat primary motor cortex. J. Neurosci. 25, 1579–1587 (2005).
Herfst, L.J. & Brecht, M. Whisker movements evoked by stimulation of single motor neurons in the facial nucleus of the rat. J. Neurophysiol. 99, 2821–2832 (2008).
Martin, M.R. & Lodge, D. Morphology of the facial nucleus of the rat. Brain Res. 123, 1–12 (1977).
Dörfl, J. The musculature of the mystacial vibrissae of the white mouse. J. Anat. 135, 147–154 (1982).
Berndt, A., Yizhar, O., Gunaydin, L.A., Hegemann, P. & Deisseroth, K. Bi-stable neural state switches. Nat. Neurosci. 12, 229–234 (2009).
Berndt, A. et al. High-efficiency channelrhodopsins for fast neuronal stimulation at low light levels. Proc. Natl. Acad. Sci. USA 108, 7595–7600 (2011).
Shichida, Y., Matuoka, S., Hidaka, Y. & Yoshizawa, T. Absorption spectra of intermediates of bacteriorhodopsin measured by laser photolysis at room temperatures. Biochim. Biophys. Acta 723, 240–246 (1983).
Kato, H.E. et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 482, 369–374 (2012).
Xu, H.T., Pan, F., Yang, G. & Gan, W.B. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat. Neurosci. 10, 549–551 (2007).
Hauss-Wegrzyniak, B., Lynch, M.A., Vraniak, P.D. & Wenk, G.L. Chronic brain inflammation results in cell loss in the entorhinal cortex and impaired LTP in perforant path-granule cell synapses. Exp. Neurol. 176, 336–341 (2002).
Grutzendler, J., Kasthuri, N. & Gan, W.B. Long-term dendritic spine stability in the adult cortex. Nature 420, 812–816 (2002).
Sohler, T.P., Lothrop, G.N. & Forbes, H.S. The poial circulation of normal, non-anesthetized animals. Part 1. Description of a method of observation. J. Pharmacol. Exp. Ther. 71, 325–330 (1941).
Drew, P.J. et al. Chronic optical access through a polished and reinforced thinned skull. Nat. Methods 7, 981–984 (2010).
Gradinaru, V. et al. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J. Neurosci. 27, 14231–14238 (2007).
Osakada, F. et al. New rabies virus variants for monitoring and manipulating activity and gene expression in defined neural circuits. Neuron 71, 617–631 (2011).
Armstrong, C.M. & Gilly, W.F. Access resistance and space clamp problems associated with whole-cell patch clamping. Methods Enzymol. 207, 100–122 (1992).
Knutsen, P.M., Derdikman, D. & Ahissar, E. Tracking whisker and head movements in unrestrained behaving rodents. J. Neurophysiol. 93, 2294–2301 (2005).
Acknowledgements
J.Y.L. was funded by the Foundation of Research, Science and Technology New Zealand, and P.M.K. was supported by a long-term fellowship from the Human Frontier Science Program (HFSP). The project was supported by grants to R.Y.T. from the US National Institutes of Health (NS027177) and the Howard Hughes Medical Institute and grants to D.K. from the US National Institutes of Health (DA029706, OD006831 and NS058668). We thank P. Tsai for suggesting the name ReaChR. We thank K. Deisseroth (Stanford University) for the hChR2H134R-eYFP construct, K. Svoboda (Howard Hughes Medical Institute Janelia Farm Research Campus) for the CAG promoter vector, E. Boyden (Massachusetts Institute of Technology) for the lentiviral vector, L. Tian (Howard Hughes Medical Institute Janelia Farm Research Campus) for the AAV2 vector and D. Trono (École Polytechnique Fédérale De Lausane) for the psPAX2 and pMD2.G lentivirus packaging vectors. AAV2-ReaChR-Citrine and pLenti-ReaChR-Citrine constructs can be requested from http://tsienlab.ucsd.edu/Samples.htm.
Author information
Authors and Affiliations
Contributions
J.Y.L. designed and developed ReaChR and conducted and analyzed the experiments in HEK293 and neuron cultures. P.M.K. and A.M. conducted and analyzed the in vivo experiments. D.K. and R.Y.T. contributed to the design and analysis of the experiments. All authors contributed to the writing and discussions of the manuscripts.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-10 (PDF 3215 kb)
Supplementary Data Set
Supplementary Data Set 1 (XLS 172 kb)
Supplementary Video 1
Example of whisker protraction evoked by inter-aural stimulation of a mouse expressing ReaChR in FN motoneurons. Movements were captured with high-speed video (800 fps), during isoflurane anesthesia, and tracked offline. FN was stimulated by placing a 617 nm LED at the opening of the external auditory canal (100 mW light output; red vertical bars; see Methods). Upon photo-stimulation the whiskers promptly protracted and remained protracted for the duration of the light pulse (100 msec). The whiskers then retracted to their initial reference position. (MOV 1115 kb)
Supplementary Video 2
Example of whisker retraction evoked by inter-aural stimulation of a mouse expressing ReaChR in FN motoneurons. Movements were captured with high-speed video (800 fps), and whisker position and angle tracked offline. As the mouse recovered from isoflurane anesthesia, the whiskers slowly protracted and remained in a protracted position for approximately one minute. During this time window, ReaChR expressing neurons in FN were stimulated by inter-aural illumination with a 617 nm LED (100 mW light output; red vertical bars). Upon photo-activation, the whiskers promptly retracted and remained retracted for the duration of the light pulse (100 msec). As the light turned off, the whiskers then returned to their initial protracted position. (MOV 1556 kb)
Rights and permissions
About this article
Cite this article
Lin, J., Knutsen, P., Muller, A. et al. ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation. Nat Neurosci 16, 1499–1508 (2013). https://doi.org/10.1038/nn.3502
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3502
This article is cited by
-
Bioinspired nanotransducers for neuromodulation
Nano Research (2024)
-
Impact of volume and expression time in an AAV-delivered channelrhodopsin
Molecular Brain (2023)
-
A Drosophila heart optical coherence microscopy dataset for automatic video segmentation
Scientific Data (2023)
-
Ultrafast light targeting for high-throughput precise control of neuronal networks
Nature Communications (2023)
-
A micro-LED array based platform for spatio-temporal optogenetic control of various cardiac models
Scientific Reports (2023)