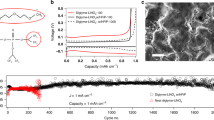
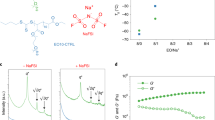
Abstract
Electrochemical energy storage is one of the main societal challenges of this century. The performances of classical lithium-ion technology based on liquid electrolytes have made great advances in the past two decades, but the intrinsic instability of liquid electrolytes results in safety issues. Solid polymer electrolytes would be a perfect solution to those safety issues, miniaturization and enhancement of energy density. However, as in liquids, the fraction of charge carried by lithium ions is small (<20%), limiting the power performances. Solid polymer electrolytes operate at 80 °C, resulting in poor mechanical properties and a limited electrochemical stability window. Here we describe a multifunctional single-ion polymer electrolyte based on polyanionic block copolymers comprising polystyrene segments. It overcomes most of the above limitations, with a lithium-ion transport number close to unity, excellent mechanical properties and an electrochemical stability window spanning 5 V versus Li+/Li. A prototype battery using this polyelectrolyte outperforms a conventional battery based on a polymer electrolyte.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Tollefson, J. Car industry: Charging up the future. Nature 456, 436–440 (2008).
Cheng, F., Liang, J., Tao, Z. & Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 23, 1695–1715 (2011).
Armand, M. & Tarascon, J-M. Building better batteries. Nature 451, 652–657 (2008).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J-M. Li–O2 and Li–S batteries with high energy storage. Nature Mater. 11, 19–29 (2012).
Murata, K., Izuchi, S. & Yoshihisa, Y. An overview of the research and development of solid polymer electrolyte batteries. Electrochim. Acta 45, 1501–1508 (2000).
Bruce, P. G. & Vincent, C. A. Polymer electrolytes. J. Chem. Soc. Faraday Trans. 89, 3187–3203 (1993).
Chazalviel, J-N. Electrochemical aspects of the generation of ramified metallic electrodeposits. Phys. Rev. A 42, 7355–7367 (1990).
Ryu, S. W. et al. Effect of counter ion placement on conductivity in single-ion conducting block copolymer electrolytes. J. Electrochem. Soc. 152, A158–A163 (2005).
Goodenough, J. B. & Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 22, 587–603 (2010).
Hammami, A., Raymond, N. & Armand, M. Lithium-ion batteries: Runaway risk of forming toxic compounds. Nature 424, 635–636 (2003).
Marzantowicz, M., Dygas, J. R., Krok, F., Florjańczyk, Z. & Zygadło-Monikowska, E. Influence of crystalline complexes on electrical properties of PEO:LiTFSI electrolyte. Electrochim. Acta 53, 1518–1526 (2007).
Vaia, R. A., Vasudevan, S., Krawiec, W., Scanlon, L. G. & Giannelis, E. P. New polymer electrolyte nanocomposites: Melt intercalation of poly(ethylene oxide) in mica-type silicates. Adv. Mater. 7, 154–156 (1995).
Wong, S. & Zax, D. B. What do NMR linewidths tell us? Dynamics of alkali cations in a PEO-based nanocomposite polymer electrolyte. Electrochim. Acta 42, 3513–3518 (1997).
Bujdàk, J., Hackett, E. & Giannelis, E. P. Effect of layer charge on the intercalation of poly(ethylene oxide) in layered silicates: Implications on nanocomposite polymer electrolytes. Chem. Mater. 12, 2168–2174 (2000).
Capiglia, C., Mustarelli, P., Quartarone, E., Tomasi, C. & Magistris, A. Effects of nanoscale SiO2 on the thermal and transport properties of solvent-free, poly(ethylene oxide) (PEO)-based polymer electrolytes. Solid State Ion. 118, 73–79 (1999).
Croce, F., Appetecchi, G. B., Persi, L. & Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 394, 456–458 (1998).
Forsyth, M. et al. The effect of nano-particle TiO2 fillers on structure and transport in polymer electrolytes. Solid State Ion. 147, 203–211 (2002).
Manuel Stephan, A. Review on gel polymer electrolytes for lithium batteries. Eur. Polymer J. 42, 21–42 (2006).
Dollé, M., Sannier, L., Beaudoin, B., Trentin, M. & Tarascon, J-M. Live scanning electron microscope observations of dendritic growth in lithium/polymer cells. Electrochem. Solid-State Lett. 5, A286–A289 (2002).
Rosso, M. et al. Dendrite short-circuit and fuse effect on Li/polymer/Li cells. Electrochim. Acta 51, 5334–5340 (2006).
Singh, M. et al. Effect of molecular weight on the mechanical and electrical properties of block copolymer electrolytes. Macromolecules 40, 4578–4585 (2007).
Panday, A. et al. Effect of molecular weight and salt concentration on conductivity of block copolymer electrolytes. Macromolecules 42, 4632–463 (2009).
Soo, P. P. et al. Rubbery block copolymer electrolytes for solid-state rechargeable lithium batteries. J. Electrochem. Soc. 146, 32–37 (1999).
Trapa, P. E., Huang, B., Won, Y-Y., Sadoway, D. R. & Mayes, A. M. Block copolymer electrolytes synthesized by atom transfer radical polymerization for solid-state, thin-film lithium batteries. Electrochem. Solid-State Lett. 5, A85–A88 (2002).
Trapa, P. E. et al. Rubbery graft copolymer electrolytes for solid-state, thin-film lithium batteries. J. Electrochem. Soc. 152, A1–A5 (2005).
Sadoway, D. R. Block and graft copolymer electrolytes for high-performance, solid-state, lithium batteries. J. Power Sources 129, 1–3 (2004).
Niitani, T. et al. Synthesis of Li+ ion conductive PEO-PSt block copolymer electrolyte with microphase separation structure. Electrochem. Solid-State Lett. 8, A385–A388 (2005).
Hayamizu, K., Akiba, E., Bando, T. & Aihara, Y. 1H, 7Li, and 19F nuclear magnetic resonance and ionic conductivity studies for liquid electrolytes composed of glymes and polyetheneglycol dimethyl ethers of CH3O(CH2CH2O)nCH3 (n = 3–50) doped with LiN(SO2CF3)2 . J. Chem. Phys. 117, 5929–5939 (2002).
Mayes, A., Sadoway, D. R. & Bannerjee, P. Block copolymer electrolyte. Patent WO 00/05774 (2000).
Sadoway, D. R. et al. Self-doped block copolymer electrolytes for solid-state, rechargeable lithium batteries. J. Power Sources 97–98, 621–623 (2001).
Hu, Q. et al. Graft copolymer-based lithium-ion battery for high-temperature operation. J. Power Sources 196, 5604–5610 (2011).
Meyer, W. H. Polymer electrolytes for lithium-ion batteries. Adv. Mater. 10, 439–448 (1998).
Abetz, V. & Simon, P. F. W. Phase behaviour and morphologies of block copolymers. Adv. Polym. Sci. 189, 125–212 (2005).
MacDonald, J. R. Binary electrolyte small-signal frequency response. Electroanal. Chem. Int. Electrochem. 53, 1–55 (1974).
Wu, D. Y., Meure, S. & Solomon, D. Self-healing polymeric materials: A review of recent developments. Prog. Polym. Sci. 33, 479–522 (2008).
Siqueira, L. J. A. & Ribeiro, M. C. C. Molecular dynamics simulation of the polymer electrolyte poly(ethyleneoxide)/LiClO4. I. Structural properties. J. Chem. Phys. 122, 194911 (2005).
Armand, M. Polymer solid electrolytes—an overview. Solid State Ion. 9–10, 745–754 (1983).
Croce, F., Sacchetti, S. & Scrosati, B. Advanced, lithium batteries based on high-performance composite polymer electrolytes. J. Power Sources 162, 685–689 (2006).
Wang, L., Li, X. & Yang, W. Enhancement of electrochemical properties of hot-pressed poly(ethylene oxide)-based nanocomposite polymer electrolyte films for all-solid-state lithium polymer batteries. Electrochim. Acta 55, 1895–1899 (2010).
Kaneko, F. et al. Capacity fading mechanism in all solid-state lithium polymer secondary batteries using PEG-borate/aluminate ester as plasticizer for polymer electrolytes. Adv. Funct. Mater. 19, 918–925 (2009).
Meziane, R., Bonnet, J-P., Courty, M., Djellab, K. & Armand, M. Single-ion polymer electrolytes based on a delocalized polyanion for lithium batteries. Electrochim. Acta 57, 14–19 (2011).
Bloch, E., Phan, T., Bertin, D., Llewellyn, P. & Hornebecq, V. Direct synthesis of mesoporous silica presenting large and tunable pores using BAB triblock copolymers: Influence of each copolymer block on the porous structure. Micropor. Mesopor. Mater. 112, 612–620 (2008).
Girod, M., Phan, T. N. T. & Charles, L. Microstructural study of a nitroxide-mediated poly(ethylene oxide)/polystyrene block copolymer (PEO-b-PS) by electrospray tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 19, 1163–1175 (2008).
Gigmes, D. et al. Intermolecular radical 1,2-addition of the BlocBuilder MA alkoxyamine onto activated olefins: A versatile tool for the synthesis of complex macromolecular architecture. Polym. Chem. 2, 1624–1631 (2011).
Acknowledgements
The present work was undertaken within the French ANR programme STOCK-E under the contract COPOLIBAT, no. ANR-09-STOCK-E-03.
Author information
Authors and Affiliations
Contributions
R.B. conceived and designed the material. R.M., L.L., J-P.B. and M.A. performed the synthesis and characterization of the anionic monomers. S.M., L.L., T.N.T.P., D.G. and D.B. performed the synthesis and macromolecular characterization of the polyanionic block copolymers. S.M. and R.D. carried out the thermal analysis. A.A., D.D., R.D. and. R.B. performed the sample and battery preparation, the conductivity and mechanical measurements and electrochemical characterizations. R.B. analysed the data and wrote the paper. All authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information (PDF 814 kb)
Rights and permissions
About this article
Cite this article
Bouchet, R., Maria, S., Meziane, R. et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nature Mater 12, 452–457 (2013). https://doi.org/10.1038/nmat3602
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nmat3602
This article is cited by
-
Functionally antagonistic polyelectrolyte for electro-ionic soft actuator
Nature Communications (2024)
-
Polyimide covalent organic frameworks as efficient solid-state Li+ electrolytes
Science China Chemistry (2024)
-
The Promise of 3D Printed Solid Polymer Electrolytes for Developing Sustainable Batteries: A Techno-Commercial Perspective
International Journal of Precision Engineering and Manufacturing-Green Technology (2024)
-
A review of solid-state lithium metal batteries through in-situ solidification
Science China Chemistry (2024)
-
Bioinspired design of Na-ion conduction channels in covalent organic frameworks for quasi-solid-state sodium batteries
Nature Communications (2023)