Abstract
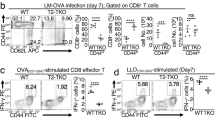
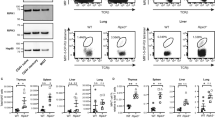
The kinase TAK1 is critical for innate and B cell immunity. The function of TAK1 in T cells is unclear, however. We show here that T cell–specific deletion of the gene encoding TAK1 resulted in reduced development of thymocytes, especially of regulatory T cells expressing the transcription factor Foxp3. In mature thymocytes, TAK1 was required for interleukin 7–mediated survival and T cell receptor–dependent activation of transcription factor NF-κB and the kinase Jnk. In effector T cells, TAK1 was dispensable for T cell receptor–dependent NF-κB activation and cytokine production, but was important for proliferation and activation of the kinase p38 in response to interleukins 2, 7 and 15. Thus, TAK1 is essential for the integration of T cell receptor and cytokine signals to regulate the development, survival and function of T cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Dong, C., Davis, R.J. & Flavell, R.A. MAP kinases in immune response. Annu. Rev. Immunol. 20, 55–72 (2002).
Ghosh, S. & Karin, M. Missing pieces in the NF-κB puzzle. Cell 109, S81–S96 (2002).
Kyriakis, J.M. & Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807–869 (2001).
Huang, Q. et al. Differential regulation of interleukin 1 receptor and Toll-like receptor signaling by MEKK3. Nat. Immunol. 5, 98–103 (2004).
Shim, J.H. et al. TAK1, but not TAB1 or TAB2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 19, 2668–2681 (2005).
Sato, S. et al. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 6, 1087–1095 (2005).
Ninomiya-Tsuji, J. et al. The kinase TAK1 can activate the NIK-IκB as well as the MAP kinase cascade in the IL-1 signalling pathway. Nature 398, 252–256 (1999).
Takaesu, G. et al. TAK1 is critical for IκB kinase-mediated activation of the NF-κB pathway. J. Mol. Biol. 326, 105–115 (2003).
Yang, J. et al. The essential role of MEKK3 in TNF-induced NF-κB activation. Nat. Immunol. 2, 620–624 (2001).
Vidal, S. et al. Mutations in the Drosophila dTAK1 gene reveal a conserved function for MAPKKKs in the control of rel/NF-κB-dependent innate immune responses. Genes Dev. 15, 1900–1912 (2001).
Shinohara, H. et al. PKCβ regulates BCR-mediated IKK activation by facilitating the interaction between TAK1 and CARMA1. J. Exp. Med. 202, 1423–1431 (2005).
Sun, L., Deng, L., Ea, C.-K., Xia, Z.-P. & Chen, Z.J. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 (2004).
Ruland, J. & Mak, T.W. Transducing signals from antigen receptors to nuclear factor κB. Immunol. Rev. 193, 93–100 (2003).
Thome, M. CARMA1, BCL-10 and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4, 348–359 (2004).
Weil, R. & Israel, A. T-cell-receptor- and B-cell-receptor-mediated activation of NF-κB in lymphocytes. Curr. Opin. Immunol. 16, 374–381 (2004).
Karin, M. & Lin, A. NF-κB at the crossroads of life and death. Nat. Immunol. 3, 221–227 (2002).
Siebenlist, U., Brown, K. & Claudio, E. Control of lymphocyte development by nuclear factor-κB. Nat. Rev. Immunol. 5, 435–445 (2005).
Lee, P.P. et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity 15, 763–774 (2001).
Sen, J. et al. Expression and induction of nuclear factor-κB-related proteins in thymocytes. J. Immunol. 154, 3213–3221 (1995).
Sabapathy, K. et al. c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J. Exp. Med. 193, 317–328 (2001).
Schmidt-Supprian, M. et al. Mature T cells depend on signaling through the IKK complex. Immunity 19, 377–389 (2003).
Pasparakis, M., Schmidt-Supprian, M. & Rajewsky, K. IκB kinase signaling Is essential for maintenance of mature B cells. J. Exp. Med. 196, 743–752 (2002).
Rincon, M. et al. The JNK pathway regulates the in vivo deletion of immature CD4+CD8+ thymocytes. J. Exp. Med. 188, 1817–1830 (1998).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Fontenot, J.D. & Rudensky, A.Y. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat. Immunol. 6, 331–337 (2005).
Fry, T.J. & Mackall, C.L. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J. Immunol. 174, 6571–6576 (2005).
Ruland, J. & Mak, T.W. From antigen to activation: specific signal transduction pathways linking antigen receptors to NF-κB. Semin. Immunol. 15, 177–183 (2003).
Vella, A.T., Dow, S., Potter, T.A., Kappler, J. & Marrack, P. Cytokine-induced survival of activated T cells in vitro and in vivo. Proc. Natl. Acad. Sci. USA 95, 3810–3815 (1998).
Schluns, K.S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nat. Rev. Immunol. 3, 269–279 (2003).
Kovanen, P.E. & Leonard, W.J. Cytokines and immunodeficiency diseases: critical roles of the γc-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol. Rev. 202, 67–83 (2004).
Sears, R.C. & Nevins, J.R. Signaling networks that link cell proliferation and cell fate. J. Biol. Chem. 277, 11617–11620 (2002).
Sherr, C.J. & Roberts, J.M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
DeGregori, J. The Rb network. J. Cell Sci. 117, 3411–3413 (2004).
Crawley, J.B. et al. T cell proliferation in response to interleukins 2 and 7 requires p38MAP kinase activation. J. Biol. Chem. 272, 15023–15027 (1997).
Geginat, J., Sallusto, F. & Lanzavecchia, A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 194, 1711–1719 (2001).
Sen, J. et al. Intrathymic signals in thymocytes are mediated by p38 mitogen-activated protein kinase. J. Immunol. 156, 4535–4538 (1996).
Schmidt-Supprian, M. et al. Differential dependence of CD4+CD25+ regulatory and natural killer-like T cells on signals leading to NF-κB activation. Proc. Natl. Acad. Sci. USA 101, 4566–4571 (2004).
Fontenot, J.D., Rasmussen, J.P., Gavin, M.A. & Rudensky, A.Y. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6, 1142–1151 (2005).
Acknowledgements
We thank C.B. Wilson for CD4-Cre–transgenic mice, E. Eynon for discussions and F. Manzo for secretarial assistance. Supported by the National Institutes of Health (R.A.F.), the Howard Hughes Medical Institute (R.A.F.), the Cancer Research Institute (Y.Y.W.), the Arthritis National Research Foundation (H.C.) and the Charles H. Hood Foundation (H.C.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Phenotypic analysis of DP and CD4 SP thymocytes. (PDF 98 kb)
Supplementary Fig. 2
Surface expression of IL-7Rα is not altered on TAK1 deficient cells. (PDF 24 kb)
Supplementary Fig. 3
TAK1 deficiency results in accelerated T cell apoptosis in the long-term culture. (PDF 34 kb)
Supplementary Fig. 4
TAK1-deficient effector T cells compete poorly with wild-type counterparts in response to cytokine stimulation. (PDF 378 kb)
Supplementary Fig. 5
The numbers of cells recovered at the end of [3H]thymidine incorporation are comparable between TAK1 intact and deficient effector T cells. (PDF 367 kb)
Supplementary Fig. 6
TAK1 deletion did not affect the expression of CD25, IL-7Rα or IL-15Rα on effector T cells. (PDF 29 kb)
Supplementary Fig. 7
TAK1 is essential for the constitutive p38 activity in thymocytes. (PDF 85 kb)
Rights and permissions
About this article
Cite this article
Wan, Y., Chi, H., Xie, M. et al. The kinase TAK1 integrates antigen and cytokine receptor signaling for T cell development, survival and function. Nat Immunol 7, 851–858 (2006). https://doi.org/10.1038/ni1355
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1355
This article is cited by
-
TAK1 is essential for MAIT cell development and the differentiation of MAIT1 and MAIT17
Cellular & Molecular Immunology (2023)
-
Reversal of prolonged obesity-associated cerebrovascular dysfunction by inhibiting microglial Tak1
Nature Neuroscience (2020)
-
Myeloid-specific TAK1 deletion results in reduced brain monocyte infiltration and improved outcomes after stroke
Journal of Neuroinflammation (2018)
-
MAP3K7 is recurrently deleted in pediatric T-lymphoblastic leukemia and affects cell proliferation independently of NF-κB
BMC Cancer (2018)
-
MALT1 is an intrinsic regulator of regulatory T cells
Cell Death & Differentiation (2017)