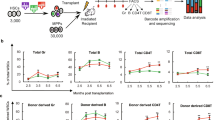
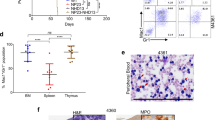
Abstract
The thymus is seeded via the blood, but the identity of hematopoietic progenitors with access to the circulation in adult mice is unknown. We report here that the only progenitors in blood with efficient T lineage potential were lineage negative with high expression of stem cell antigen 1 and c-Kit (LSK cells). The blood LSK population, like its counterpart in the bone marrow, contained hematopoietic stem cells and nonrenewing, multipotent progenitors, including early lymphoid progenitors and CD62L+ cells previously described as efficient T lineage progenitors. Common lymphoid progenitors could not be identified in the circulation, suggesting they are not physiological T lineage precursors. We conclude that blood LSK cells are the principal circulating progenitors with T lineage potential.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Morrison, S.J., Uchida, N. & Weissman, I.L. The biology of hematopoietic stem cells. Annu. Rev. Cell. Dev. Biol. 11, 35–71 (1995).
Miller, J.F. & Osoba, D. Current concepts of the immunological function of the thymus. Physiol. Rev. 47, 437–520 (1967).
Goldschneider, I., Komschlies, K.L. & Greiner, D.L. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J. Exp. Med. 163, 1–17 (1986).
Scollay, R., Smith, J. & Stauffer, V. Dynamics of early T cells: prothymocyte migration and proliferation in the adult mouse thymus. Immunol. Rev. 91, 129–157 (1986).
Donskoy, E. & Goldschneider, I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J. Immunol. 148, 1604–1612 (1992).
Foss, D.L., Donskoy, E. & Goldschneider, I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J. Exp. Med. 193, 365–374 (2001).
Petrie, H. Cell migration and the control of post-natal T-cell lymphopoiesis in the thymus. Nat. Rev. Immunol. 3, 859–866 (2003).
Bhandoola, A., Sambandam, A., Allman, D., Meraz, A. & Schwarz, B. Early T lineage progenitors: new insights, but old questions remain. J. Immunol. 171, 5653–5658 (2003).
Spangrude, G.J., Heimfeld, S. & Weissman, I.L. Purification and characterization of mouse hematopoietic stem cells. Science 241, 58–62 (1988).
Adolfsson, J. et al. Upregulation of Flt3 expression within the bone marrow Lin− Sca1+ c- kit+ stem cell compartment is accompanied by loss of self-renewal capacity. Immunity 15, 659–669 (2001).
Christensen, J.L. & Weissman, I.L. Flk-2 is a marker in hematopoietic stem cell differentiation: a simple method to isolate long-term stem cells. Proc. Natl. Acad. Sci. USA 98, 14541–14546 (2001).
Kondo, M., Weissman, I.L. & Akashi, K. Identification of clonogenic common lymphoid progenitors in mouse bone marrow. Cell 91, 661–672 (1997).
Spangrude, G.J. & Scollay, R. Differentiation of hematopoietic stem cells in irradiated mouse thymic lobes. Kinetics and phenotype of progeny. J. Immunol. 145, 3661–3668 (1990).
Allman, D. et al. Thymopoiesis independent of common lymphoid progenitors. Nat. Immunol. 4, 168–174 (2003).
Igarashi, H., Gregory, S., Yokota, T., Sakaguchi, N. & Kincade, P. Transcription from the RAG1 locus marks the earliest lymphocyte progenitors in bone marrow. Immunity 17, 117–130 (2002).
Perry, S.S. et al. L-selectin defines a bone marrow analog to the thymic early T-lineage progenitor. Blood 103, 2990–2996 (2004).
Rodewald, H.R., Kretzschmar, K., Takeda, S., Hohl, C. & Dessing, M. Identification of pro-thymocytes in murine fetal blood: T lineage commitment can precede thymus colonization. EMBO J. 13, 4229–4240 (1994).
Delassus, S. & Cumano, A. Circulation of hematopoietic progenitors in the mouse embryo. Immunity 4, 97–106 (1996).
Dunon, D., Allioli, N., Vainio, O., Ody, C. & Imhof, B.A. Quantification of T-cell progenitors during ontogeny: thymus colonization depends on blood delivery of progenitors. Blood 93, 2234–2243 (1999).
Ikawa, T. et al. Identification of the earliest prethymic T-cell progenitors in murine fetal blood. Blood 103, 530–537 (2004).
Yamamoto, Y. et al. Characterization of peripheral blood stem cells in mice. Blood 88, 445–454 (1996).
Harrison, D.E. & Astle, C.M. Short- and long-term multilineage repopulating hematopoietic stem cells in late fetal and newborn mice: models for human umbilical cord blood. Blood 90, 174–181 (1997).
To, L.B., Haylock, D.N., Simmons, P.J. & Juttner, C.A. The biology and clinical uses of blood stem cells. Blood 89, 2233–2258 (1997).
Papayannopoulou, T. Hematopoietic stem/progenitor cell mobilization: a continuing quest for etiologic mechanisms. Ann. NY Acad. Sci. 872, 187–199 (1999).
Lapidot, T. & Petit, I. Current understanding of stem cell mobilization: the roles of chemokines proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp. Hematol. 30, 973–981 (2002).
Goodman, J.W. & Hodgson, G.S. Evidence for stem cells in the peripheral blood of mice. Blood 19, 702–714 (1962).
Wright, D.E., Wagers, A.J., Gulati, A.P., Johnson, F.L. & Weissman, I.L. Physiological migration of hematopoietic stem and progenitor cells. Science 294, 1933–1936 (2001).
Wallis, V.J., Leuchars, E., Chwalinski, S. & Davies, A.J. On the sparse seeding of bone marrow and thymus in radiation chimaeras. Transplantation 19, 2–11 (1975).
Okada, S. et al. Enrichment and characterization of murine hematopoietic stem cells that express c-kit molecule. Blood 78, 1706–1712 (1991).
Ikuta, K. & Weissman, I.L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc. Natl. Acad. Sci. USA 89, 1502–1506 (1992).
Izon, D. et al. A common pathway for dendritic cell and early B cell development. J. Immunol. 167, 1387–1392 (2001).
Sitnicka, E. et al. Key role of flt3 ligand in regulation of the common lymphoid progenitor but not in maintenance of the hematopoietic stem cell pool. Immunity 17, 463–472 (2002).
Donskoy, E., Foss, D. & Goldschneider, I. Gated importation of prothymocytes by adult mouse thymus is coordinated with their periodic mobilization from bone marrow. J. Immunol. 171, 3568–3575 (2003).
Peters, L.L. et al. Large-scale, high-throughput screening for coagulation and hematologic phenotypes in mice. Physiol. Genomics 11, 185–193 (2002).
Akashi, K., Traver, D., Miyamoto, T. & Weissman, I.L. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 404, 193–197 (2000).
Gounari, F. et al. Tracing lymphopoiesis with the aid of a pTα-controlled reporter gene. Nat. Immunol. 3, 489–496 (2002).
Martin, C.H. et al. Efficient thymic immigration of B220+ lymphoid-restricted bone marrow cells with T precursor potential. Nat. Immunol. 4, 866–873 (2003).
Yokota, T. et al. Unique properties of fetal lymphoid progenitors identified according to RAG1 gene expression. Immunity 19, 365–375 (2003).
Kadish, J.L. & Basch, R.S. Hematopoietic thymocyte precursors. I. Assay and kinetics of the appearance of progeny. J. Exp. Med. 143, 1082–1099 (1976).
Allman, D. et al. Resolution of three nonproliferative immature splenic B cell subsets reveals multiple selection points during peripheral B cell maturation. J. Immunol. 167, 6834–6840 (2001).
Na Nakorn, T., Traver, D., Weissman, I.L. & Akashi, K. Myeloerythroid-restricted progenitors are sufficient to confer radioprotection and provide the majority of day 8 CFU-S. J. Clin. Invest. 109, 1579–1585 (2002).
Shortman, K. & Wu, L. Early T lymphocyte progenitors. Annu. Rev. Immunol. 14, 29–47 (1996).
Porritt, H.E., Gordon, K. & Petrie, H.T. Kinetics of steady-state differentiation and mapping of intrathymic-signaling environments by stem cell transplantation in nonirradiated mice. J. Exp. Med. 198, 957–962 (2003).
Montecino-Rodriguez, E. & Dorshkind, K. To T or not to T: reassessing the common lymphoid progenitor. Nat. Immunol. 4, 100–101 (2003).
Egerton, M., Scollay, R. & Shortman, K. Kinetics of mature T-cell development in the thymus. Proc. Natl. Acad. Sci. USA 87, 2579–2582 (1990).
Chu, V. et al. Method for non-invasively recording electrocardiograms in conscious mice. BMC Physiol. 1, 6 (2001).
Yang, B., Larson, D.F. & Watson, R. Age-related left ventricular function in the mouse: analysis based on in vivo pressure-volume relationships. Am. J. Physiol. Heart. Circ. Physiol. 277, H1906–H1913 (1999).
Fleming, W.H., Alpern, E.J., Uchida, N., Ikuta, K. & Weissman, I.L. Steel factor influences the distribution and activity of murine hematopoietic stem cells in vivo. Proc. Natl. Acad. Sci. USA 90, 3760–3764 (1993).
Heissig, B. et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109, 625–637 (2002).
Pear, W.S. & Radtke, F. Notch signaling in lymphopoiesis. Semin. Immunol. 15, 69–79 (2003).
Yu, W. et al. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature 400, 682–687 (1999).
Acknowledgements
We thank G. Spangrude, D. Allman, W. Pear, I. Maillard, R. Gerstein and D. Fonseca for critical comments; and R. Schretzenmair and H. Pletcher of the Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource (Philadelphia, Pennsylvania) for technical support. Supported by National Institutes of Health (AI059621; T32-AI-055428) and Concern Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Schwarz, B., Bhandoola, A. Circulating hematopoietic progenitors with T lineage potential. Nat Immunol 5, 953–960 (2004). https://doi.org/10.1038/ni1101
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni1101
This article is cited by
-
RBPJ-dependent Notch signaling initiates the T cell program in a subset of thymus-seeding progenitors
Nature Immunology (2019)
-
Thymus Colonization: Who, How, How Many?
Archivum Immunologiae et Therapiae Experimentalis (2018)
-
Trap1a is an X-linked and cell-intrinsic regulator of thymocyte development
Cellular & Molecular Immunology (2017)
-
Interleukin‐7 in the transition of bone marrow progenitors to the thymus
Immunology & Cell Biology (2017)
-
microRNA-449a modulates medullary thymic epithelial cell differentiation
Scientific Reports (2017)