Abstract
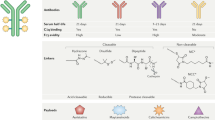
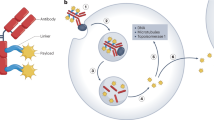
The successful introduction of genetically engineered human and chimeric immunoglobulin proteins has established monoclonal antibodies (mAbs) as a validated approach for treating malignancies. The unique properties of mAb therapies including their high affinity and specificity, and the differential expression of target antigen in tumor cells versus normal cells make them attractive agents for cancer immunotherapy. The field of immunoconjugate development attempts to combine the specificity of mAb therapies with cytotoxic and radionuclide molecules, thereby combining the best characteristics of these two different modalities. Two radiolabeled mAbs, 90Y-ibritumomab tiuxetan and 131I-tositumomab, and one drug conjugate, gemtuzumab ozogamicin have been approved for the treatment of malignancies. Other conjugates carrying toxic payloads of calicheamicin, geldanamycin, maytansinoids and taxoids as well as peptide exotoxins are undergoing preclinical and clinical development. Nevertheless, several obstacles have limited robust antitumor activity and broad application of imunoconjugates, including the optimization of three structural components of the immunoconjugate (i.e. mAb and target specificity, chemical linker design, and the cytotoxin), as well as issues common to mAb therapy such as heterogeneous antigen expression, which can limit uniform antibody delivery. This Review examines optimal design, the lessons learned from clinical immunoconjugate development, and the promising agents in early preclinical/clinical development for the treatment of cancer.
Key Points
-
Immunoconjugates should be viewed as sophisticated delivery systems for antitumor cytotoxic and radionuclide moieties
-
Successful immunoconjugate development requires consideration and optimization of the three major components of the molecule including antibody/antigen target, linker, and payload
-
Biodistribution analysis with radioimmunoconjugates, approved cytotoxic immunoconjugates, or those in development indicate that antigen on circulating cells or circulating shed antigen can profoundly influence toxicity and reduce efficacy
-
Phase II studies indicate that a cytotoxic immunoconjugate (gemtuzumab ozogamicin) can elicit clinically meaningful complete response rates in AML
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Köhler G and Milstein C (1975) Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256: 495–497
Zangemeister-Wittke U (2005) Antibodies for targeted cancer therapy—technical aspects and clinical perspectives. Pathobiology 72: 279–286
Mendelsohn J and Baselga J (2003) Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol 21: 2787–2799
Arnould L et al. (2006) Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br J Cancer 94: 259–267
Bleeker WK et al. (2004) Dual mode of action of a human anti-epidermal growth factor receptor monoclonal antibody for cancer therapy. J Immunol 173: 4699–4707
Iannello A and Ahmad A (2005) Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anti-cancer monoclonal antibodies. Cancer Metastasis Rev 24: 487–499
Rowinsky EK et al. (2004) Safety, pharmacokinetics, and activity of ABX-EGF, a fully human anti-epidermal growth factor receptor monoclonal antibody in patients with metastatic renal cell cancer [see comment]. J Clin Oncol 22: 3003–3015
Law CL et al. (2006) Lymphocyte activation antigen CD70 expressed by renal cell carcinoma is a potential therapeutic target for anti-CD70 antibody-drug conjugates. Cancer Res 66: 2328–2337
Park JW et al. (2002) Anti-HER2 immunoliposomes: enhanced efficacy attributable to targeted delivery. Clin Cancer Res 8: 1172–1181
Sjogren HO et al. (1997) Antitumor activity of carcinoma-reactive BR96-doxorubicin conjugate against human carcinomas in athymic mice and rats and syngeneic rat carcinomas in immunocompetent rats. Cancer Res 57: 4530–4536
Trail PA et al. (1997) Effect of linker variation on the stability, potency, and efficacy of carcinoma-reactive BR64-doxorubicin immunoconjugates. Cancer Res 57: 100–105
Trail PA et al. (1993) Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science 261: 212–215
Friedman PN et al. (1993) Antitumor activity of the single-chain immunotoxin BR96 sFv-PE40 against established breast and lung tumor xenografts. J Immunol 150: 3054–3061
Friedman PN et al. (1993) BR96 sFv-PE40, a potent single-chain immunotoxin that selectively kills carcinoma cells. Cancer Res 53: 334–339
Trail PA et al. (1992) Antigen-specific activity of carcinoma-reactive BR64-doxorubicin conjugates evaluated in vitro and in human tumor xenograft models. Cancer Res 52: 5693–5700
Hellstrom I et al. (1990) Highly tumor-reactive, internalizing, mouse monoclonal antibodies to Le(y)-related cell surface antigens. Cancer Res 50: 2183–2190
Saleh MN et al. (2000) Phase I trial of the anti-Lewis Y drug immunoconjugate BR96-doxorubicin in patients with lewis Y-expressing epithelial tumors. J Clin Oncol 18: 2282–2292
Tolcher AW et al. (1999) Randomized phase II study of BR96-doxorubicin conjugate in patients with metastatic breast cancer. J Clin Oncol 17: 478–484
Ajani JA et al. (2000) A multi-institutional phase II study of BMS-182248-01 (BR96-doxorubicin conjugate) administered every 21 days in patients with advanced gastric adenocarcinoma [see comment]. Cancer J 6: 78–81
Knox SJ et al. (1996) Yttrium-90-labeled anti-CD20 monoclonal antibody therapy of recurrent B-cell lymphoma. Clin Cancer Res 2: 457–470
van der Velden VH et al. (2004) High CD33-antigen loads in peripheral blood limit the efficacy of gemtuzumab ozogamicin (Mylotarg) treatment in acute myeloid leukemia patients. Leukemia 18: 983–988
Pupa SM et al. (1993) The extracellular domain of the c-erbB-2 oncoprotein is released from tumor cells by proteolytic cleavage. Oncogene 8: 2917–2923
Christianson TA et al. (1998) NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res 58: 5123–5129
Baeckstrom D et al. (1991) Purification and characterization of a membrane-bound and a secreted mucin-type glycoprotein carrying the carcinoma-associated sialyl-Lea epitope on distinct core proteins. J Biol Chem 266: 21537–21547
Erickson HK et al. (2006) Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 66: 4426–4433
Kovtun YV et al. (2006) Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res 66: 3214–3221
Wu AM and Senter PD (2005) Arming antibodies: prospects and challenges for immunoconjugates. Nat Biotechnol 23: 1137–1146
Chakrabarti MC et al. (1996) Prevention of radiolysis of monoclonal antibody during labeling. J Nucl Med 37: 1384–1388
Schnell R et al. (2002) A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin's and non-Hodgkin's lymphoma. Clin Cancer Res 8: 1779–1786
Kreitman RJ et al. (2005) Phase I trial of recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) in patients with B-cell malignancies. J Clin Oncol 23: 6719–6729
Posey JA et al. (2002) A phase I trial of the single-chain immunotoxin SGN-10 (BR96 sFv-PE40) in patients with advanced solid tumors. Clin Cancer Res 8: 3092–3099
Schnell R et al. (2003) Clinical evaluation of ricin A-chain immunotoxins in patients with Hodgkin's lymphoma. Ann Oncol 14: 729–736
Frankel AE et al. (2002) Diphtheria toxin conjugate therapy of cancer. Cancer Chemother Biol Response Modif 20: 301–313
Olsnes S (2004) The history of ricin, abrin and related toxins. Toxicon 44: 361–370
Tsimberidou AM et al. (2003) Anti-B4 blocked ricin post chemotherapy in patients with chronic lymphocytic leukemia—long-term follow-up of a monoclonal antibody-based approach to residual disease. Leuk Lymphoma 44: 1719–1725
Pastan I et al. (2006) Immunotoxin therapy of cancer. Nat Rev Cancer 6: 559–565
Uesugi M and Sugiura Y (1992) DNA cleaving modes in minor groove of DNA helix by esperamicin and calicheamicin antitumor antibiotics. Nucleic Acids Symp Ser 1–2
Hinman LM et al. (1993) Preparation and characterization of monoclonal antibody conjugates of the calicheamicins: a novel and potent family of antitumor antibiotics. Cancer Res 53: 3336–3342
Salzberg AA and Dedon PC (2000) DNA bending is a determinant of calicheamicin target recognition. Biochemistry 39: 7605–7612
DiJoseph JF et al. (2004) Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 103: 1807–1814
Woyke T et al. (2001) In vitro activities and postantifungal effects of the potent dolastatin 10 derivative auristatin PHE. Antimicrob Agents Chemother 45: 3580–3584
Mohammad RM et al. (1999) A new tubulin polymerization inhibitor, auristatin PE, induces tumor regression in a human Waldenstrom's macroglobulinemia xenograft model. Int J Oncol 15: 367–372
Francisco JA et al. (2003) cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood 102: 1458–1465
Doronina SO et al. (2006) Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconj Chem 17: 114–124
Sutherland MS et al. (2006) Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem 281: 10540–10547
Law CL et al. (2004) Efficient elimination of B-lineage lymphomas by anti-CD20-auristatin conjugates. Clin Cancer Res 10: 7842–7851
Doronina SO et al. (2003) Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 21: 778–784
Smith LM et al. (2006) Potent cytotoxicity of an auristatin-containing antibody-drug conjugate targeting melanoma cells expressing melanotransferrin/p97. Mol Cancer Ther 5: 1474–1482
Tse KF et al. (2006) CR011, a fully human monoclonal antibody-auristatin E conjugate, for the treatment of melanoma. Clin Cancer Res 12: 1373–1382
Afar DE et al. (2004) Preclinical validation of anti-TMEFF2-auristatin E-conjugated antibodies in the treatment of prostate cancer. Mol Cancer Ther 3: 921–932
Bhaskar V et al. (2003) E-selectin up-regulation allows for targeted drug delivery in prostate cancer. Cancer Res 63: 6387–6394
Widdison WC et al. (2006) Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem 49: 4392–4408
Wang L et al. (2005) Structural characterization of the maytansinoid-monoclonal antibody immunoconjugate, huN901-DM1, by mass spectrometry. Protein Sci 14: 2436–2446
Liu Z et al. (2005) Metabolism studies of the anti-tumor agent maytansine and its analog ansamitocin P-3 using liquid chromatography/tandem mass spectrometry. J Mass Spectrom 40: 389–399
Tassone P et al. (2004) Cytotoxic activity of the maytansinoid immunoconjugate B-B4-DM1 against CD138+ multiple myeloma cells. Blood 104: 3688–3696
Henry MD et al. (2004) A prostate-specific membrane antigen-targeted monoclonal antibody-chemotherapeutic conjugate designed for the treatment of prostate cancer. Cancer Res 64: 7995–8001
Helft PR et al. (2004) A phase I study of cantuzumab mertansine administered as a single intravenous infusion once weekly in patients with advanced solid tumors. Clin Cancer Res 10: 4363–4368
Tassone P et al. (2004) In vitro and in vivo activity of the maytansinoid immunoconjugate huN901-N2′-deacetyl-N2′-(3-mercapto-1-oxopropyl)-maytansine against CD56+ multiple myeloma cells. Cancer Res 64: 4629–4636
Ranson M and Sliwkowski MX (2002) Perspectives on anti-HER monoclonal antibodies. Oncology 63 (Suppl 1): 17–24
Sievers EL et al. (1999) Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93: 3678–3684
Nabhan C et al. (2004) Gemtuzumab ozogamicin (MylotargTM) is infrequently associated with sinusoidal obstructive syndrome/veno-occlusive disease. Ann Oncol 15: 1231–1236
Larson RA et al. (2005) Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 104: 1442–1452
Dowell JA et al. (2001) Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol 41: 1206–1214
Sievers EL et al. (2001) Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J Clin Oncol 19: 3244–3254
Korth-Bradley JM et al. (2001) Impact of age and gender on the pharmacokinetics of gemtuzumab ozogamicin. Pharmacotherapy 21: 1175–1180
van Der Velden VH et al. (2001) Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 97: 3197–3204
Walter RB et al. (2005) Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood 105: 1295–1302
Jedema I et al. (2004) Internalization and cell cycle-dependent killing of leukemic cells by Gemtuzumab Ozogamicin: rationale for efficacy in CD33-negative malignancies with endocytic capacity. Leukemia 18: 316–325
Linenberger ML et al. (2001) Multidrug-resistance phenotype and clinical responses to gemtuzumab ozogamicin. Blood 98: 988–994
Sievers EL (2001) Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukaemia in first relapse. Exp Opin Biol Ther 1: 893–901
Bross PF et al. (2001) Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res 7: 1490–1496
Wadleigh M et al. (2003) Prior gemtuzumab ozogamicin exposure significantly increases the risk of veno-occlusive disease in patients who undergo myeloablative allogeneic stem cell transplantation. Blood 102: 1578–1582
Arceci RJ et al. (2005) Safety and efficacy of gemtuzumab ozogamicin in pediatric patients with advanced CD33+ acute myeloid leukemia. Blood 106: 1183–1188
Buckwalter M et al. (2004) Pharmacokinetics of gemtuzumab ozogamicin as a single-agent treatment of pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Pharmacol 44: 873–880
Leopold LH et al. (2003) Comparative efficacy and safety of gemtuzumab ozogamicin monotherapy and high-dose cytarabine combination therapy in patients with acute myeloid leukemia in first relapse. Clin Adv Hematol Oncol 1: 220–225
Piccaluga PP et al. (2004) First experience with gemtuzumab ozogamicin plus cytarabine as continuous infusion for elderly acute myeloid leukaemia patients. Leukemia Res 28: 987–990
Kell WJ et al. (2003) A feasibility study of simultaneous administration of gemtuzumab ozogamicin with intensive chemotherapy in induction and consolidation in younger patients with acute myeloid leukemia. Blood 102: 4277–4283
Swords R et al. (2004) Successful induction of molecular remission with single-agent anti-CD33 antibody (gemtuzumab ozogamicin) in chemotherapy-refractory relapse of acute myeloid leukaemia post-BMT. Eur J Haematol 73: 450–451
Lutz R et al. (2005) HuC242-DM4, an antibody maytansinoid conjugate with superior preclinical activity in human CanAg -positive tumor xenografts in SCID mice. Proc Am Assoc Cancer Res A1429
Ojima I et al. (2002) Tumor-specific novel taxoid-monoclonal antibody conjugates. J Med Chem 45: 5620–5623
Carter P (2001) Improving the efficacy of antibody-based cancer therapies. Nat Rev Cancer 1: 118–12955
Acknowledgements
We thank Susan J Knox et al., Clinical Cancer Research and IDEC Pharmaceuticals Corporation for the provision and permission to use Figure 2.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declared that they have no competing financial interests. AD Ricart and AW Tolcher are investigators in clinical trials with immunoconjugates and Dr. Tolcher has acted as a consultant role to Biogen IDEC, and Genentech.
Rights and permissions
About this article
Cite this article
Ricart, A., Tolcher, A. Technology Insight: cytotoxic drug immunoconjugates for cancer therapy. Nat Rev Clin Oncol 4, 245–255 (2007). https://doi.org/10.1038/ncponc0774
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/ncponc0774
This article is cited by
-
Antibody drug conjugates as targeted cancer therapy: past development, present challenges and future opportunities
Archives of Pharmacal Research (2023)
-
Specific targeting of HER2-positive human breast carcinoma SK-BR-3 cells by amygdaline-ZHER2 affibody conjugate
Molecular Biology Reports (2020)
-
Drug Development of Therapeutic Monoclonal Antibodies
BioDrugs (2016)
-
Synthesis of novel 5-methyl pyrazol-3-one derivatives and their in vitro cytotoxic evaluation
Medicinal Chemistry Research (2015)
-
CD30 as a Therapeutic Target for Lymphoma
BioDrugs (2014)