Abstract
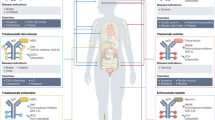
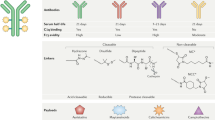
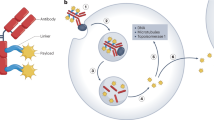
Advances in our understanding of cancer biology have enabled drug development to progress towards better targeted therapies that are both more effective and safer owing to their lack of off-target toxicities. In this regard, antibody–drug conjugates (ADCs), which have the potential to combine the selectivity of therapeutic antibodies with the cytotoxicity of highly toxic small molecules, are a rapidly developing drug class. The complex and unique structure of an ADC, composed of a monoclonal antibody conjugated to a potent cytotoxic payload via a chemical linker, is designed to selectively target a specific tumour antigen. The success of an ADC is highly dependent on the specific properties of its components, all of which have implications for the stability, cytotoxicity, pharmacokinetics and antitumour activity of the ADC. The development of therapeutic ADCs, including gemtuzumab ozogamicin and inotuzumab ozogamicin, provided great knowledge of the refinements needed for the optimization of such agents. In this Review, we describe the key components of ADC structure and function and focus on the clinical development and subsequent utilization of two leukaemia-directed ADCs — gemtuzumab ozogamicin and inotuzumab ozogamicin — as well as on the mechanisms of resistance and predictors of response to these two agents.
Key points
-
The development of antibody–drug conjugates (ADCs) has enabled the targeted delivery of potent cytotoxins to cancer cells while also limiting the extent of off-target effects.
-
The structural components of ADCs include a monoclonal antibody, a linker and a payload, all of which affect the antitumour activity, toxicity and pharmacokinetics to varying degrees.
-
Gemtuzumab ozogamicin, for patients with acute myeloid leukaemia, was the first ADC to be approved and has paved the way for the emergence of several novel ADCs in both haematological malignancies and solid tumours.
-
Inotuzumab ozogamicin has significantly improved the outcomes of patients with acute lymphoid leukaemia both as a single agent and when administered in combination with HCVD, in patients with acute lymphoid leukaemia compared with cytotoxic chemotherapy alone
-
Expression of drug efflux pumps and/or single nucleotide polymorphisms can reduce the efficacy of ADCs
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ehrlich, P. Address in Pathology, on Chemotherapy: delivered before the Seventeenth International Congress of Medicine. Br. Med. J. 2, 353–359 (1913).
Di Gaetano, N. et al. Complement activation determines the therapeutic activity of rituximab in vivo. J. Immunol. 171, 1581–1587 (2003).
Taylor, R. P. & Lindorfer, M. A. The role of complement in mAb-based therapies of cancer. Methods 65, 18–27 (2014).
Mathe, G., Tran Ba, L. O. & Bernard, J. Effect on mouse leukemia 1210 of a combination by diazo-reaction of amethopterin and gamma-globulins from hamsters inoculated with such leukemia by heterografts. C. R. Hebd. Seances Acad. Sci. 246, 1626–1628 (1958).
Ghose, T., Cerini, M., Carter, M. & Nairn, R. C. Immunoradioactive agent against cancer. Br. Med. J. 1, 90–93 (1967).
Ghose, T. & Nigam, S. P. Antibody as carrier of chlorambucil. Cancer 29, 1398–1400 (1972).
Rowland, G. F., O’Neill, G. J. & Davies, D. A. Suppression of tumour growth in mice by a drug-antibody conjugate using a novel approach to linkage. Nature 255, 487–488 (1975).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Ford, C. H. et al. Localisation and toxicity study of a vindesine-anti-CEA conjugate in patients with advanced cancer. Br. J. Cancer 47, 35–42 (1983).
Nabhan, C. & Tallman, M. S. Early phase I/II trials with gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia. Clin. Lymphoma 2 (Suppl. 1), S19–S23 (2002).
Nabhan, C. et al. Phase II pilot trial of gemtuzumab ozogamicin (GO) as first line therapy in acute myeloid leukemia patients age 65 or older. Leuk. Res. 29, 53–57 (2005).
Petersdorf, S. H. et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood 121, 4854–4860 (2013).
Ravandi, F. et al. Gemtuzumab ozogamicin: time to resurrect? J. Clin. Oncol. 30, 3921–3923 (2012).
Sievers, E. L. et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 19, 3244–3254 (2001).
Giles, F. J. et al. Mylotarg (gemtuzumab ozogamicin) therapy is associated with hepatic venoocclusive disease in patients who have not received stem cell transplantation. Cancer 92, 406–413 (2001).
Castaigne, S. et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 379, 1508–1516 (2012).
Burnett, A. K. et al. The addition of gemtuzumab ozogamicin to intensive chemotherapy in older patients with AML produces a significant improvement in overall survival: results of the UK NCRI AML16 randomized trial. Blood 118, 582–582 (2011).
Burnett, A. K. et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J. Clin. Oncol. 29, 369–377 (2011).
Saunders, K. O. Conceptual approaches to modulating antibody effector functions and circulation half-life. Front. Immunol. 10, 1296 (2019).
Pyzik, M. et al. The neonatal Fc receptor (FcRn): a misnomer? Front. Immunol. 10, 1540 (2019).
Mackness, B. C. et al. Antibody Fc engineering for enhanced neonatal Fc receptor binding and prolonged circulation half-life. mAbs 11, 1276–1288 (2019).
Ritchie, M., Tchistiakova, L. & Scott, N. Implications of receptor-mediated endocytosis and intracellular trafficking dynamics in the development of antibody drug conjugates. mAbs 5, 13–21 (2013).
Polson, A. G., Ho, W. Y. & Ramakrishnan, V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin. Investig. Drugs 20, 75–85 (2011).
Hamblett, K. J. et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin. Cancer Res. 10, 7063–7070 (2004).
Lucas, A. T. et al. Factors affecting the pharmacology of antibody-drug conjugates. Antibodies 7, 10 (2018).
Sun, X. et al. Effects of drug-antibody ratio on pharmacokinetics, biodistribution, efficacy, and tolerability of antibody-maytansinoid conjugates. Bioconjug. Chem. 28, 1371–1381 (2017).
King, H. D. et al. Monoclonal antibody conjugates of doxorubicin prepared with branched peptide linkers: inhibition of aggregation by methoxytriethyleneglycol chains. J. Med. Chem. 45, 4336–4343 (2002).
Mehrling, T. & Soltis, D. Challenges in optimising the successful construction of antibody drug conjugates in cancer therapy. Antibodies 7, 11 (2018).
Harding, F. A., Stickler, M. M., Razo, J. & DuBridge, R. B. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. mAbs 2, 256–265 (2010).
Hwang, W. Y. & Foote, J. Immunogenicity of engineered antibodies. Methods 36, 3–10 (2005).
Rosenberg, A. S. Immunogenicity of biological therapeutics: a hierarchy of concerns. Dev. Biol. 112, 15–21 (2003).
Ducry, L. & Stump, B. Antibody-drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 21, 5–13 (2010).
Satomaa, T. et al. Hydrophilic auristatin glycoside payload enables improved antibody-drug conjugate efficacy and biocompatibility. Antibodies 7, 15 (2018).
Dhakal, D., Yogesh, D. & Sohng, J. K. Book Review: Antibody–drug conjugates: fundametnals, drug development, and clinical outcomes to target cancer. Front. Pharmacol. 8, 771 (2017).
Doronina, S. O. et al. Enhanced activity of monomethylauristatin F through monoclonal antibody delivery: effects of linker technology on efficacy and toxicity. Bioconjug Chem. 17, 114–124 (2006).
Lu, J., Jiang, F., Lu, A. & Zhang, G. Linkers having a crucial role in antibody-drug conjugates. Int. J. Mol. Sci. 17, 561 (2016).
Teicher, B. A. & Chari, R. V. Antibody conjugate therapeutics: challenges and potential. Clin. Cancer Res. 17, 6389–6397 (2011).
Li, F. et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res. 76, 2710–2719 (2016).
Byun, J. H. & Jung, I. H. Modeling to capture bystander-killing effect by released payload in target positive tumor cells. BMC Cancer 19, 194 (2019).
Barok, M., Joensuu, H. & Isola, J. Trastuzumab emtansine: mechanisms of action and drug resistance. Breast Cancer Res. 16, 209 (2014).
Yardley, D. A. et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer previously treated with chemotherapy and 2 or more HER2-targeted agents: results from the T-PAS expanded access study. Cancer J. 21, 357–364 (2015).
Zein, N., Sinha, A. M., McGahren, W. J. & Ellestad, G. A. Calicheamicin gamma 1I: an antitumor antibiotic that cleaves double-stranded DNA site specifically. Science 240, 1198–1201 (1988).
Smellie, M., Kelland, L. R., Thurston, D. E., Souhami, R. L. & Hartley, J. A. Cellular pharmacology of novel C8-linked anthramycin-based sequence-selective DNA minor groove cross-linking agents. Br. J. Cancer 70, 48–53 (1994).
Jenkins, T. C., Hurley, L. H., Neidle, S. & Thurston, D. E. Structure of a covalent DNA minor groove adduct with a pyrrolobenzodiazepine dimer: evidence for sequence-specific interstrand cross-linking. J. Med. Chem. 37, 4529–4537 (1994).
Hurley, L. H. et al. Pyrrolo[1,4]benzodiazepine antitumor antibiotics: relationship of DNA alkylation and sequence specificity to the biological activity of natural and synthetic compounds. Chem. Res. Toxicol. 1, 258–268 (1988).
Pemmaraju, N. et al. Tagraxofusp in blastic plasmacytoid dendritic-cell neoplasm. N. Engl. J. Med. 380, 1628–1637 (2019).
Schnell, R. et al. A Phase I study with an anti-CD30 ricin A-chain immunotoxin (Ki-4.dgA) in patients with refractory CD30+ Hodgkin’s and non-Hodgkin’s lymphoma. Clin. Cancer Res. 8, 1779–1786 (2002).
Madhumathi, J., Devilakshmi, S., Sridevi, S. & Verma, R. S. Immunotoxin therapy for hematologic malignancies: where are we heading? Drug Discov. Today 21, 325–332 (2016).
Akbari, B. et al. Immunotoxins in cancer therapy: Review and update. Int. Rev. Immunol. 36, 207–219 (2017).
van Der Velden, V. H. et al. Targeting of the CD33-calicheamicin immunoconjugate Mylotarg (CMA-676) in acute myeloid leukemia: in vivo and in vitro saturation and internalization by leukemic and normal myeloid cells. Blood 97, 3197–3204 (2001).
Bross, P. F. et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin. Cancer Res. 7, 1490–1496 (2001).
Sievers, E. L. et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93, 3678–3684 (1999).
Hibma, J. & Knight, B. Population pharmacokinetic modeling of gemtuzumab ozogamicin in adult patients with acute myeloid leukemia. Clin. Pharmacokinet. 58, 335–347 (2019).
Food and Drug Administration. ODAC Briefing Document BLA 761060 from the Oncologic Drugs Advisory Committee Meeting on July 11/2017 regarding Mylotarg (gemtuzumab ozogamicin). FDA, https://www.fda.gov/media/106500/download (2017).
Larson, R. A. et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer 104, 1442–1452 (2005).
Lowenberg, B. et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N. Engl. J. Med. 361, 1235–1248 (2009).
Luskin, M. R. et al. Benefit of high-dose daunorubicin in AML induction extends across cytogenetic and molecular groups. Blood 127, 1551–1558 (2016).
Burnett, A. K. et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60 mg/m2 in AML induction: results from the UK NCRI AML17 trial in 1206 patients. Blood 125, 3878–3885 (2015).
Lambert, J. et al. Gemtuzumab ozogamicin for de novo acute myeloid leukemia: final efficacy and safety updates from the open-label, phase III ALFA-0701 trial. Haematologica 104, 113–119 (2019).
Burnett, A. K. et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J. Clin. Oncol. 30, 3924–3931 (2012).
Hills, R. K. et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 15, 986–996 (2014).
Gamis, A. S. et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J. Clin. Oncol. 32, 3021–3032 (2014).
Estey, E. H. et al. Experience with gemtuzumab ozogamycin (“mylotarg”) and all-trans retinoic acid in untreated acute promyelocytic leukemia. Blood 99, 4222–4224 (2002).
Lo-Coco, F. et al. Gemtuzumab ozogamicin (Mylotarg) as a single agent for molecularly relapsed acute promyelocytic leukemia. Blood 104, 1995–1999 (2004).
Breccia, M. et al. Sustained molecular remission after low dose gemtuzumab-ozogamicin in elderly patients with advanced acute promyelocytic leukemia. Haematologica 92, 1273–1274 (2007).
Takeshita, A. et al. Efficacy of gemtuzumab ozogamicin on ATRA- and arsenic-resistant acute promyelocytic leukemia (APL) cells. Leukemia 19, 1306–1311 (2005).
Abaza, Y. et al. Long-term outcome of acute promyelocytic leukemia treated with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab. Blood 129, 1275–1283 (2017).
Lancet, J. E. et al. A phase 2 study of ATRA, arsenic trioxide, and gemtuzumab ozogamicin in patients with high-risk APL (SWOG 0535). Blood Adv. 4, 1683–1689 (2020).
National Comprehensive Cancer Network. Acute myeloid leukemia. NCCN, https://www.nccn.org/professionals/physician_gls/pdf/aml.pdf (2020).
Borthakur, G. M. et al. Fludarabine, cytarabine, G-CSF and gemtuzumab ozogamicin (FLAG-GO) regimen results in better molecular response and relapse-free survival in core binding factor acute myeloid leukemia than FLAG and idarubicin (FLAG-Ida). Blood 134, 290–290 (2019).
Lambert, J. et al. MRD assessed by WT1 and NPM1 transcript levels identifies distinct outcomes in AML patients and is influenced by gemtuzumab ozogamicin. Oncotarget 5, 6280–6288 (2014).
Kapp-Schwoerer, S. et al. Impact of gemtuzumab ozogamicin on MRD and relapse risk in NPM1 mutated AML patients: results from the AMLSG 09-09 Trial. Blood 136, 3041–3050 (2020).
Olejniczak, S. H., Stewart, C. C., Donohue, K. & Czuczman, M. S. A quantitative exploration of surface antigen expression in common B-cell malignancies using flow cytometry. Immunol. Invest. 35, 93–114 (2006).
DiJoseph, J. F. et al. Antibody-targeted chemotherapy of B-cell lymphoma using calicheamicin conjugated to murine or humanized antibody against CD22. Cancer Immunol. Immunother. 54, 11–24 (2005).
DiJoseph, J. F. et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood 103, 1807–1814 (2004).
Advani, A. et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J. Clin. Oncol. 28, 2085–2093 (2010).
Ogura, M. et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci. 101, 1840–1845 (2010).
Goy, A. et al. A phase 2 study of inotuzumab ozogamicin in patients with indolent B-cell non-Hodgkin lymphoma refractory to rituximab alone, rituximab and chemotherapy, or radioimmunotherapy. Br. J. Haematol. 174, 571–581 (2016).
Fayad, L. et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J. Clin. Oncol. 31, 573–583 (2013).
Dijoseph, J. F., Dougher, M. M., Armellino, D. C., Evans, D. Y. & Damle, N. K. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia 21, 2240–2245 (2007).
Betts, A. M. et al. Preclinical to clinical translation of antibody-drug conjugates using PK/PD modeling: a retrospective analysis of inotuzumab ozogamicin. AAPS J. 18, 1101–1116 (2016).
DeAngelo, D. J. et al. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 1, 1167–1180 (2017).
Kantarjian, H. et al. Inotuzumab ozogamicin, an anti-CD22-calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: a phase 2 study. Lancet Oncol. 13, 403–411 (2012).
Kantarjian, H. et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer 119, 2728–2736 (2013).
Kantarjian, H. M. et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N. Engl. J. Med. 375, 740–753 (2016).
Jabbour, E. et al. Impact of salvage treatment phase on inotuzumab ozogamicin treatment for relapsed/refractory acute lymphoblastic leukemia: an update from the INO-VATE final study database. Leuk. Lymphoma 61, 2012–2015 (2020).
Kantarjian, H. M. et al. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: Final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer 125, 2474–2487 (2019).
Jabbour, E. et al. Impact of minimal residual disease status in patients with relapsed/refractory acute lymphoblastic leukemia treated with inotuzumab ozogamicin in the phase III INO-VATE trial. Leuk. Res. 88, 106283 (2020).
Marks, D. I. et al. Outcomes of allogeneic stem cell transplantation after inotuzumab ozogamicin treatment for relapsed or refractory acute lymphoblastic leukemia. Biol. Blood Marrow Transpl. 25, 1720–1729 (2019).
Jabbour, E. et al. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 clinical trial. JAMA Oncol. 4, 230–234 (2018).
Jabbour, E. et al. Chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD, with or without blinatumomab, is highly effective in patients with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer 124, 4044–4055 (2018).
Jabbour, E. J. et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: A propensity score analysis. Cancer 125, 2579–2586 (2019).
Jain, N. et al. Inotuzumab ozogamicin with bosutinib in relapsed or refractory philadelphia chromosome-positive (PH+) acute lymphoblastic leukemia (ALL) or chronic myeloid leukemia in lymphoid blast phase (CML-LBP) [Abstract EP396]. Presented at European Hematology Association (EHA) held in Frankfurt, Germany, on 12 June 2020, https://library.ehaweb.org/eha/2020/eha25th/294315/nitin.jain.inotuzumab.ozogamicin.with.bosutinib.in.relapsed.or.refractory.html (2020).
Guffroy, M. et al. Liver microvascular injury and thrombocytopenia of antibody-calicheamicin conjugates in cynomolgus monkeys-mechanism and monitoring. Clin. Cancer Res. 23, 1760–1770 (2017).
Ho, V. T. et al. Prior gemtuzumab ozogamicin exposure in adults with acute myeloid leukemia does not increase hepatic veno-occlusive disease risk after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research analysis. Biol. Blood Marrow Transpl. 26, 884–892 (2020).
Stock, W. et al. Efficacy of inotuzumab ozogamicin in patients with Philadelphia chromosome-positive relapsed/refractory acute lymphoblastic leukemia. Cancer https://doi.org/10.1002/cncr.33321 (2020).
Taksin, A. L. et al. High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 21, 66–71 (2007).
Richardson, P. G. et al. Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood 127, 1656–1665 (2016).
Richardson, P. G. et al. Defibrotide for the treatment of severe hepatic veno-occlusive disease and multiorgan failure after stem cell transplantation: a multicenter, randomized, dose-finding trial. Biol. Blood Marrow Transpl. 16, 1005–1017 (2010).
Gloude, N. J. et al. Combination of high-dose methylprednisolone and defibrotide for veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Biol. Blood Marrow Transpl. 24, 91–95 (2018).
Myers, K. C., Lawrence, J., Marsh, R. A., Davies, S. M. & Jodele, S. High-dose methylprednisolone for veno-occlusive disease of the liver in pediatric hematopoietic stem cell transplantation recipients. Biol. Blood Marrow Transpl. 19, 500–503 (2013).
Ng, S. W. K. et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature 540, 433–437 (2016).
Ng, S. W. K. et al. A novel predictor of response to gemtuzumab ozogamicin therapy in AML provides strategies for sensitization of leukemia stem cells in individual patients. Blood 132, 2765–2765 (2018).
Pollard, J. A. et al. CD33 expression and its association with gemtuzumab ozogamicin response: results from the randomized phase III children’s oncology group trial AAML0531. J. Clin. Oncol. 34, 747–755 (2016).
Olombel, G. et al. The level of blast CD33 expression positively impacts the effect of gemtuzumab ozogamicin in patients with acute myeloid leukemia. Blood 127, 2157–2160 (2016).
Khan, N. et al. Expression of CD33 is a predictive factor for effect of gemtuzumab ozogamicin at different doses in adult acute myeloid leukaemia. Leukemia 31, 1059–1068 (2017).
Lamba, J. K. et al. CD33 splicing polymorphism determines gemtuzumab ozogamicin response in De novo acute myeloid leukemia: report from randomized phase III children’s oncology group Trial AAML0531. J. Clin. Oncol. 35, 2674–2682 (2017).
Gale, R. E. et al. No evidence that CD33 splicing SNP impacts the response to GO in younger adults with AML treated on UK MRC/NCRI trials. Blood 131, 468–471 (2018).
Jabbour, E. et al. Prognostic factors for outcome in patients with refractory and relapsed acute lymphocytic leukemia treated with inotuzumab ozogamicin, a CD22 monoclonal antibody. Am. J. Hematol. 90, 193–196 (2015).
Jabbour, E. et al. Prognostic implications of cytogenetics in adults with acute lymphoblastic leukemia treated with inotuzumab ozogamicin. Am. J. Hematol. 94, 408–416 (2019).
Kantarjian, H. M. et al. Efficacy and safety outcomes in the phase 3 INO-vate trial by baseline CD22 positivity assessed by local laboratories. Blood 134, 1344–1344 (2019).
Kebriaei, P. et al. Exploration of potential relationships between CD22 and selected Safety outcomes in the inotuzumab ozogamicin phase 3 INO-VATE study. Blood 132, 4031–4031 (2018).
Shah, N. N. et al. Evaluation of CD22 modulation as a mechanism of resistance to inotuzumab ozogamicin (InO): results from central CD22 testing on the Children’s Oncology Group (COG) phase II trial of INO in children and young adults with CD22+ B-acute lymphoblastic leukemia (B-ALL). J. Clin. Oncol. 38, 10519–10519 (2020).
Garcia-Alonso, S., Ocana, A. & Pandiella, A. Resistance to antibody-drug conjugates. Cancer Res. 78, 2159–2165 (2018).
Cianfriglia, M., Mallano, A., Ascione, A. & Dupuis, M. L. Multidrug transporter proteins and cellular factors involved in free and mAb linked calicheamicin-gamma1 (gentuzumab ozogamicin, GO) resistance and in the selection of GO resistant variants of the HL60 AML cell line. Int. J. Oncol. 36, 1513–1520 (2010).
Walter, R. B. et al. Multidrug resistance protein attenuates gemtuzumab ozogamicin-induced cytotoxicity in acute myeloid leukemia cells. Blood 102, 1466–1473 (2003).
Matsui, H. et al. Reduced effect of gemtuzumab ozogamicin (CMA-676) on P-glycoprotein and/or CD34-positive leukemia cells and its restoration by multidrug resistance modifiers. Leukemia 16, 813–819 (2002).
Takeshita, A. et al. CMC-544 (inotuzumab ozogamicin) shows less effect on multidrug resistant cells: analyses in cell lines and cells from patients with B-cell chronic lymphocytic leukaemia and lymphoma. Br. J. Haematol. 146, 34–43 (2009).
Amin, M. L. P-glycoprotein inhibition for optimal drug delivery. Drug Target. Insights 7, 27–34 (2013).
Rafiee, R. et al. ABCB1 SNP predicts outcome in patients with acute myeloid leukemia treated with Gemtuzumab ozogamicin: a report from Children’s Oncology Group AAML0531 Trial. Blood Cancer J. 9, 51 (2019).
Stein, E. M. et al. A phase 1 trial of vadastuximab talirine as monotherapy in patients with CD33-positive acute myeloid leukemia. Blood 131, 387–396 (2018).
Fathi, A. T. et al. A phase 1 trial of vadastuximab talirine combined with hypomethylating agents in patients with CD33-positive AML. Blood 132, 1125–1133 (2018).
Kovtun, Y. et al. IMGN779, a novel CD33-targeting antibody-drug conjugate with DNA-alkylating activity, exhibits potent antitumor activity in models of AML. Mol. Cancer Ther. 17, 1271–1279 (2018).
Cortes, J. E. et al. Maturing clinical profile of IMGN779, a next-generation CD33-targeting antibody-drug conjugate, in patients with relapsed or refractory acute myeloid leukemia. Blood 132, 26–26 (2018).
Narayan, R. et al. A phase 1 study of the antibody-drug conjugate brentuximab vedotin with re-induction chemotherapy in patients with CD30-expressing relapsed/refractory acute myeloid leukemia. Cancer 126, 1264–1273 (2020).
Daver, N. G. et al. Clinical profile of IMGN632, a novel CD123-targeting antibody-drug conjugate (ADC), in patients with relapsed/refractory (R/R) acute myeloid leukemia (AML) or blastic plasmacytoid dendritic cell neoplasm (BPDCN). Blood 134, 734–734 (2019).
Daver, N. G. et al. A phase 1b/2 study of the CD123-targeting antibody-drug conjugate IMGN632 As Monotherapy or in combination with venetoclax and/or azacitidine for patients with CD123-positive acute myeloid leukemia. Blood 134, 2601–2601 (2019).
Rudra-Ganguly, N., Lowe, C., Challita-Eid, P. M., Mattie, M. in AACR 574 (Springer, 2016).
Snyder, J. T. et al. Metabolism of an oxime-linked antibody drug conjugate, AGS62P1, and characterization of its identified metabolite. Mol. Pharm. 15, 2384–2390 (2018).
Amadori, S. et al. Gemtuzumab ozogamicin versus best supportive care in older patients with newly diagnosed acute myeloid leukemia unsuitable for intensive chemotherapy: results of the randomized phase III EORTC-GIMEMA AML-19 trial. J. Clin. Oncol. 34, 972–979 (2016).
Delaunay, J. et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of aml patients with intermediate cytogenetics not eligible for allogeneic transplantation. results of the GOELAMS AML 2006 IR study. Blood 118, 79–79 (2011).
Amadori, S. et al. Sequential combination of gemtuzumab ozogamicin and standard chemotherapy in older patients with newly diagnosed acute myeloid leukemia: results of a randomized phase III trial by the EORTC and GIMEMA consortium (AML-17). J. Clin. Oncol. 31, 4424–4430 (2013).
Burnett, A. et al. Defining the dose of gemtuzumab ozogamicin in combination with induction chemotherapy in acute myeloid leukemia: a comparison of 3 mg/m2 with 6 mg/m2 in the NCRI AML17 trial. Haematologica 101, 724–731 (2016).
Schlenk, R. F. et al. Gemtuzumab ozogamicin in NPM1-mutated acute myeloid leukemia: early results from the prospective randomized AMLSG 09-09 Phase III study. J. Clin. Oncol. 38, 623–632 (2020).
Advani, A. S. et al. A phase II study of weekly inotuzumab ozogamicin (InO) in adult patients with CD22-positive acute lymphoblastic leukemia (ALL) in second or later salvage. Blood 124, 2255–2255 (2014).
O’Brien, M. M. et al. A phase 2 trial of inotuzumab ozogamicin (InO) in children and young adults with relapsed or refractory (R/R) CD22+ B-acute lymphoblastic leukemia (B-ALL): results from children’s oncology group protocol AALL1621. Blood 134, 741–741 (2019).
Bhojwani, D. et al. Inotuzumab ozogamicin in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. Leukemia 33, 884–892 (2019).
Kantarjian, H. et al. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 19, 240–248 (2018).
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to all aspects of the preparation of this manuscript.
Corresponding author
Ethics declarations
Competing interests
E.J. has acted as a consultant for and received research funding from Abbvie, Amgen, Bristol-Myers Squibb, Pfizer and Takeda. H.K. has acted as a consultant for and received research funding from Abbvie, Amgen, Bristol-Myers Squibb, Pfizer and Takeda. S.P. declares no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks A. Takeshita and the other, anonymous, reviewers for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Jabbour, E., Paul, S. & Kantarjian, H. The clinical development of antibody–drug conjugates — lessons from leukaemia. Nat Rev Clin Oncol 18, 418–433 (2021). https://doi.org/10.1038/s41571-021-00484-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-021-00484-2
This article is cited by
-
Balancing speed, science and regulatory requirements in oncology drug development
Nature Medicine (2022)
-
Monomethyl auristatin antibody and peptide drug conjugates for trimodal cancer chemo-radio-immunotherapy
Nature Communications (2022)
-
Novel Approaches for the Treatment of Patients with Richter’s Syndrome
Current Treatment Options in Oncology (2022)
-
An update on antibody–drug conjugates in urothelial carcinoma: state of the art strategies and what comes next
Cancer Chemotherapy and Pharmacology (2022)