Abstract
During mitotic spindle assembly, γ-tubulin ring complexes (γTuRCs) nucleate microtubules at centrosomes, around chromosomes, and, by interaction with augmin, from pre-existing microtubules. How different populations of microtubules are organized to form a bipolar spindle is poorly understood, in part because we lack information on the dynamics of microtubule minus ends. Here we show that γTuRC is associated with minus ends of non-centrosomal spindle microtubules. Recruitment of γTuRC to spindles occurs preferentially at pole-distal regions, requires nucleation and/or interaction with minus ends, and is followed by sorting of minus-end-bound γTuRC towards the poles. Poleward movement of γTuRC exceeds k-fibre flux, involves the motors dynein, HSET (also known as KIFC1; a kinesin-14 family member) and Eg5 (also known as KIF11; a kinesin-5 family member), and slows down in pole-proximal regions, resulting in the accumulation of minus ends. Thus, in addition to nucleation, γTuRC actively contributes to spindle architecture by organizing microtubule minus ends.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Meunier, S. & Vernos, I. Microtubule assembly during mitosis — from distinct origins to distinct functions? J. Cell Sci. 125, 2805–2814 (2012).
Tirnauer, J. S., Salmon, E. D. & Mitchison, T. J. Microtubule plus-end dynamics in Xenopus egg extract spindles. Mol. Biol. Cell 15, 1776–1784 (2004).
Burbank, K. S., Mitchison, T. J. & Fisher, D. S. Slide-and-cluster models for spindle assembly. Curr. Biol. 17, 1373–1383 (2007).
Burbank, K. S., Groen, A. C., Perlman, Z. E., Fisher, D. S. & Mitchison, T. J. A new method reveals microtubule minus ends throughout the meiotic spindle. J. Cell Biol. 175, 369–375 (2006).
Brugués, J., Nuzzo, V., Mazur, E. & Needleman, D. J. Nucleation and transport organize microtubules in metaphase spindles. Cell 149, 554–564 (2012).
Loughlin, R., Heald, R. & Nedelec, F. A computational model predicts Xenopus meiotic spindle organization. J. Cell Biol. 191, 1239–1249 (2010).
Mastronarde, D. N., McDonald, K. L., Ding, R. & McIntosh, J. R. Interpolar spindle microtubules in PTK cells. J. Cell Biol. 123, 1475–1489 (1993).
Kamasaki, T. et al. Augmin-dependent microtubule nucleation at microtubule walls in the spindle. J. Cell Biol. 202, 25–33 (2013).
Goshima, G. et al. Genes required for mitotic spindle assembly in Drosophila S2 cells. Science 316, 417–421 (2007).
Goshima, G., Mayer, M., Zhang, N., Stuurman, N. & Vale, R. D. Augmin: a protein complex required for centrosome-independent microtubule generation within the spindle. J. Cell Biol. 181, 421–429 (2008).
Lawo, S. et al. HAUS, the 8-subunit human Augmin complex, regulates centrosome and spindle integrity. Curr. Biol. 19, 816–826 (2009).
Inclán, Y. F. & Nogales, E. Structural models for the self-assembly and microtubule interactions of γ-, δ- and ε-tubulin. J. Cell Sci. 114, 413–422 (2001).
Hendrickson, T. W., Yao, J., Bhadury, S., Corbett, A. H. & Joshi, H. C. Conditional mutations in γ-tubulin reveal its involvement in chromosome segregation and cytokinesis. Mol. Biol. Cell 12, 2469–2481 (2001).
Zhu, H., Coppinger, J. A., Jang, C-Y., Yates, J. R. & Fang, G. FAM29A promotes microtubule amplification via recruitment of the NEDD1-γ-tubulin complex to the mitotic spindle. J. Cell Biol. 183, 835–848 (2008).
Lüders, J., Patel, U. K. & Stearns, T. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin-mediated microtubule nucleation. Nat. Cell Biol. 8, 137–147 (2006).
Johmura, Y. et al. Regulation of microtubule-based microtubule nucleation by mammalian polo-like kinase 1. Proc. Natl Acad. Sci. USA 108, 11446–11451 (2011).
Haren, L. et al. NEDD1-dependent recruitment of the γ-tubulin ring complex to the centrosome is necessary for centriole duplication and spindle assembly. J. Cell Biol. 172, 505–515 (2006).
Manning, J. A., Shalini, S., Risk, J. M., Day, C. L. & Kumar, S. A direct interaction with NEDD1 regulates γ-tubulin recruitment to the centrosome. PLoS ONE 5, e9618 (2010).
Haren, L., Stearns, T. & Lüders, J. Plk1-dependent recruitment of γ-tubulin complexes to mitotic centrosomes involves multiple PCM components. PLoS ONE 4, e5976 (2009).
Petry, S., Groen, A. C., Ishihara, K., Mitchison, T. J. & Vale, R. D. Branching microtubule nucleation in Xenopus egg extracts mediated by augmin and TPX2. Cell 152, 768–777 (2013).
Patterson, G. H. & Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 297, 1873–1877 (2002).
Hallen, M. A., Ho, J., Yankel, C. D. & Endow, S. A. Fluorescence recovery kinetic analysis of γ-tubulin binding to the mitotic spindle. Biophys. J. 95, 3048–3058 (2008).
Yang, G., Cameron, L. A., Maddox, P. S., Salmon, E. D. & Danuser, G. Regional variation of microtubule flux reveals microtubule organization in the metaphase meiotic spindle. J. Cell Biol. 182, 631–639 (2008).
Ganem, N. J., Upton, K. & Compton, D. A. Efficient mitosis in human cells lacking poleward microtubule flux. Curr. Biol. 15, 1827–1832 (2005).
Maffini, S. et al. Motor-independent targeting of CLASPs to kinetochores by CENP-E promotes microtubule turnover and poleward flux. Curr. Biol. 19, 1566–1572 (2009).
Goshima, G., Nédélec, F. & Vale, R. D. Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. J. Cell Biol. 171, 229–240 (2005).
Cai, S., Weaver, L. N., Ems-McClung, S. C. & Walczak, C. E. Kinesin-14 family proteins HSET/XCTK2 control spindle length by cross-linking and sliding microtubules. Mol. Biol. Cell 20, 1348–1359 (2009).
Mountain, V. et al. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 147, 351–366 (1999).
Ferenz, N. P., Gable, A. & Wadsworth, P. Mitotic functions of kinesin-5. Semin. Cell Dev. Biol. 21, 255–259 (2010).
Kapitein, L. C. et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435, 114–118 (2005).
Firestone, A. J. et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484, 125–129 (2012).
Cai, S., Weaver, L. N., Ems-McClung, S. C. & Walczak, C. E. Proper organization of microtubule minus ends is needed for midzone stability and cytokinesis. Curr. Biol. 20, 880–885 (2010).
Uehara, R. & Goshima, G. Functional central spindle assembly requires de novo microtubule generation in the interchromosomal region during anaphase. J. Cell Biol. 191, 259–267 (2010).
Khodjakov, A. & Rieder, C. L. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 146, 585–596 (1999).
Goodwin, S. S. & Vale, R. D. Patronin regulates the microtubule network by protecting microtubule minus ends. Cell 143, 263–274 (2010).
Wang, H., Brust-Mascher, I., Civelekoglu-Scholey, G. & Scholey, J. M. Patronin mediates a switch from kinesin-13-dependent poleward flux to anaphase B spindle elongation. J. Cell Biol. 203, 35–46 (2013).
Hayward, D., Metz, J., Pellacani, C. & Wakefield, J. G. Synergy between multiple microtubule-generating pathways confers robustness to centrosome-driven mitotic spindle formation. Dev. Cell 28, 81–93 (2014).
Petry, S., Pugieux, C., Nédélec, F. J. & Vale, R. D. Augmin promotes meiotic spindle formation and bipolarity in Xenopus egg extracts. Proc. Natl Acad. Sci. USA 108, 14473–14478 (2011).
Leber, B. et al. Proteins required for centrosome clustering in cancer cells. Sci. Transl. Med. 2, 33ra38 (2010).
Kwon, M. et al. Mechanisms to suppress multipolar divisions in cancer cells with extra centrosomes. Genes Dev. 22, 2189–2203 (2008).
Basto, R. et al. Centrosome amplification can initiate tumorigenesis in flies. Cell 133, 1032–1042 (2008).
Kleylein-Sohn, J. et al. Acentrosomal spindle organization renders cancer cells dependent on the kinesin HSET. J. Cell Sci. 125, 5391–5402 (2013).
Steigemann, P. et al. Aurora B-mediated abscission checkpoint protects against tetraploidization. Cell 136, 473–484 (2009).
Tulu, U. S., Rusan, N. M. & Wadsworth, P. Peripheral, non-centrosome-associated microtubules contribute to spindle formation in centrosome-containing cells. Curr. Biol. 13, 1894–1899 (2003).
Nam, H-S. & Benezra, R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell 5, 515–526 (2009).
Vinopal, S. et al. γ-Tubulin 2 nucleates microtubules and is downregulated in mouse early embryogenesis. PLoS ONE 7, e29919 (2012).
Cai, S., O’Connell, C., Khodjakov, A. & Walczak, C. Chromosome congression in the absence of kinetochore fibres. Nat. Cell Biol. 11, 832–838 (2009).
Murphy, S. M. et al. GCP5 and GCP6: two new members of the human γ-tubulin complex. Mol. Biol. Cell 12, 3340–3352 (2001).
Teixidó-Travesa, N. et al. The γTuRC revisited: a comparative analysis of interphase and mitotic human γTuRC redefines the set of core components and identifies the novel subunit GCP8. Mol. Biol. Cell 21, 3963–3972 (2010).
Acknowledgements
We thank C. Lacasa for technical support, P. Draber (Institute of Molecular Genetics of the ASCR, Prague, Czech Republic) for the TUBG1 shRNA plasmid, I. Vernos (CRG, Barcelona, Spain) for anti-TPX2 antibodies, and J. Colombelli and the staff of the Advanced Digital Microscopy facility of the IRB Barcelona for their excellent support. We are particularly grateful to S. Tosi for providing custom macros for image analysis. This study was supported by grants BFU2009-08522 and BFU2012-33960 to J.L. (MINECO, Spain), a Marie Curie International Re-integration Grant (FP7-PEOPLE-2007-4-3-IRG, project #224835) to J.L., and IRB Barcelona intramural funds. J.L. acknowledges additional support from the Ramón y Cajal Programme (MINECO, Spain). N.L. was supported by a La Caixa PhD fellowship (‘la Caixa’ Foundation, Spain).
Author information
Authors and Affiliations
Contributions
N.L. and J.L. designed the study, performed experiments and analysed data. J.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Pole-proximal enrichment of γTuRC in spindles of U2OS cells and characterization of the γ-tubulin minus end binding mutant.
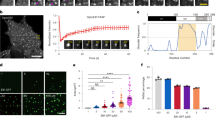
(a) Immunofluorescence microscopy images of U2OS cells fixed and stained with antibodies against γ-tubulin, NEDD1, HAUS6, and α-tubulin as indicated. DAPI was used to stain DNA. Scale bar, 5 μm. (b) Fluorescence intensities in half-spindles of U2OS cells prepared as in a were quantified along the spindle axis and means were plotted relative to the α-tubulin intensities as a function of distance from the spindle poles. For each protein the value closest to the pole was set to one (error bars, s.e.m.; n = 30 half-spindles). (c) Hela cells were transfected with plasmids expressing EGFP as a control, or expressing γ-tubulin shRNA in combination with myc-tagged γ-tubulin wild type, or myc-tagged γ-tubulin 4A mutant, respectively. After 72 h lysates were prepared and probed by western blotting with antibodies against γ-tubulin and GAPDH as loading control. (d) Mutant γTuRC in cleared lysates (Input) from cells transfected with plasmid encoding γ-tubulin shRNA and mutant γ-tubulin-4A-myc was separated from smaller molecular weight complexes by precipitation with polyethylene glycol (PEG) to obtain PEG supernatant (S) and pellet (P) fractions. The γTuRC-containing pellet was resuspended and subjected to immunoprecipitation (IP) with control IgG or anti-Myc antibody. Immunoprecipitated γTuRC was detected by western blotting with antibodies against GCP4 and γ-tubulin. Detection of GAPDH served as a control. The relative amounts of tagged γ-tubulin 4A mutant and endogenous γ-tubulin present in γTuRC, which were quantified from the bands detected by the anti-γ-tubulin antibody, are indicated. The asterisk indicates cross-reactivity with the IgG heavy chain derived from the anti-Myc antibody used for immunoprecipitation. (e) Cells were transfected as in c. Lysates were prepared and immunoprecipitated with anti-Myc antibody. Samples were probed with antibodies against the indicated proteins by western blotting. (f) Hela cells transfected with plasmids expressing γ-tubulin shRNA in combination with myc-tagged γ-tubulin wild type or myc-tagged γ-tubulin 4A mutant were fixed and stained with anti-myc and anti-pericentrin antibodies; DAPI was used to label DNA. Scale bar, 5 μm.
Supplementary Figure 2 Characterization of the NEDD1 γ-tubulin binding mutant.
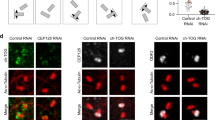
(a) Hela cells were transfected with plasmids expressing NEDD1 shRNA and EGFP-tagged NEDD1 wild type or Y643A/S644A mutant. After 48 h cells were arrested in mitosis by incubation in nocodazole for 5 h and cold treatment to depolymerize microtubules. Cells were fixed and stained with antibodies against GFP and γ-tubulin; DAPI was used to label DNA. Scale bar, 5 μm. (b) Cells were prepared as in a and for each condition the fluorescence intensities of the indicated proteins at centrosomes in the absence of microtubules were quantified. The mean intensity obtained for cells expressing wild type NEDD1-EGFP was set to one (error bars, s.e.m.; n = 25 centrosomes for wild type and n = 30 centrosome for the mutant; ∗∗∗∗P < 0.0001). (c) Cells were transfected with plasmids expressing EGFP, or NEDD1 shRNA in combination with EGFP-tagged NEDD1 wild type or EGFP tagged NEDD1 Y643A/S644A mutant, respectively. After 48 h cells were arrested in mitosis by incubation in nocodazole over night. Mitotic extracts were prepared and immunoprecipitated with anti-GFP antibody. Samples were probed with antibodies against the indicated proteins by western blotting. The asterisk indicates cross-reactivity with the IgG heavy chain derived from the anti-GFP antibody used for immunoprecipitation.
Supplementary Figure 3 Microtubule flux in U2OS metaphase spindles.
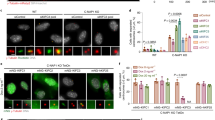
(a) Using laser-directed photoactivation a fluorescent mark was positioned perpendicular to the spindle axis in spindles of U2OS cells expressing paGFP-α-tubulin and histone H2B-mCherry and analysed by time-lapse microscopy. Labelling of chromosomes by H2B-mCherry was used to identify metaphase cells and as a visual guide for positioning the activation mark. Shown are still images of a merge of the channels for paGFP-α-tubulin (green) and H2B-mCherry (red) before and after photoactivation. Numbers below images indicate time in seconds. The yellow line in the image before photoactivation indicates the area that was targeted by the laser. Scale bar, 5 μm. (b) Representative kymographs revealing the poleward movement of paGFP-α-tubulin fluorescence in half spindles. Graphs show line scans of kymographs at the indicated time points. In addition to the bulk of fluorescence that is associated with k-fibres, there is a population of microtubules that moves poleward more rapidly. This causes an increase in fluorescence between the activation mark and the pole and is seen as a shoulder next to the fluorescence peak in the line scans. Scale bar, 1 μm. (c) Cells were transfected with control or HSET siRNA and analysed by western blotting with antibodies against HSET and GAPDH as loading control. The asterisk indicates a non-specific band recognized by the HSET antibody.
Supplementary Figure 4 Supplementary Figure Western blots
Uncropped immunoblots referring to Supplementary Figs 1c–e, 2c, and 3c. Labels on top of each membrane scan indicate the antibody used for detection. Dashed red boxes show the regions that were cropped for the figures.
Supplementary information
Supplementary Information
Supplementary Information (PDF 884 kb)
Supplementary Information
Supplementary Information (XLSX 34 kb)
Poleward transport of γ-tubulin-paGFP in control cells and in cells treated with EHNA and STLC.
γ-Tubulin-paGFP fluorescence (green) was activated in metaphase spindles of control cells and cells treated with EHNA and STLC to inhibit dynein and Eg5, respectively. Microtubules are labelled by expression of α-tubulin-mCherry (red). Poleward movement of the fluorescent marks was monitored by time-lapse microscopy. (MOV 407 kb)
Poleward transport of γ-tubulin-paGFP wild type and 4A mutant.
Cells transfected with plasmid expressing α-tubulin shRNA together wild type or mutant γ-tubulin-paGFP, and plasmid expressing γ-tubulin-mCherry were imaged by time-lapse microscopy. γ-Tubulin-paGFP fluorescence (green) was activated in metaphase spindles, which are visualized by α-tubulin-mCherry (red). (MOV 905 kb)
Poleward transport and incorporation of γ-tubulin-paGFP at centrosomes.
γ-Tubulin-paGFP-expressing cells were imaged by time-lapse microcopy. γ-Tubulin-paGFP fluorescence was activated in metaphase spindles and poleward transport was allowed to occur for two minutes. Cells were further incubated in the absence or presence of nocodazole to depolymerize spindle microtubules. (MOV 1067 kb)
Rights and permissions
About this article
Cite this article
Lecland, N., Lüders, J. The dynamics of microtubule minus ends in the human mitotic spindle. Nat Cell Biol 16, 770–778 (2014). https://doi.org/10.1038/ncb2996
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncb2996
This article is cited by
-
Mechanisms underlying spindle assembly and robustness
Nature Reviews Molecular Cell Biology (2023)
-
Sub-centrosomal mapping identifies augmin-γTuRC as part of a centriole-stabilizing scaffold
Nature Communications (2021)
-
Changes in microtubule overlap length regulate kinesin-14-driven microtubule sliding
Nature Chemical Biology (2017)
-
Mitotic spindle assembly in animal cells: a fine balancing act
Nature Reviews Molecular Cell Biology (2017)
-
Spindle pole cohesion requires glycosylation-mediated localization of NuMA
Scientific Reports (2017)