Abstract
Lymph node metastases is an important prognostic indicator for disease progression and crucial for therapeutic strategies in the work-up of colorectal carcinoma. In this study, we investigated tumor lymphangiogenesis and vascular endothelial growth factor (VEGF) expression as predictive markers for the risk of lymph node metastasis and their relation to other prognostic parameters in colorectal carcinoma. Resected colorectal carcinomas from 90 patients were examined, including 30 patients without lymph node metastases, 30 with only lymph node metastases, and 30 with liver metastases. Cases were immunostained for CD31, D2-40, and VEGF. Positivity stained microvessels were counted in densely vascular/lymphatic foci (hot spots) at × 400 field (=0.17 mm2). Intensity of staining for VEGF was scored on a two-tiered scale. D2-40 lymphatic microvessel density demonstrated significant correlation with CD31 counts (20±9 vs 18±6/0.17 mm2 field, P<0.05) and VEGF expression (P<0.01). VEGF was expressed in 61/90 (67%) cases. D2-40 identified lymphatic tumor invasion in 48/90 patients, which was greater than CD31 (37/90) and hematoxylin and eosin (H&E) (31/90). There was a positive significant correlation of D2-40, CD31 counts, and VEGF expression with the presence of lymphovascular invasion and lymph node metastases (P<0.05). D2-40 lymphatic microvessel density correlated significantly with depth of invasion (pT), positive vascular pedicle lymph nodes and liver metastases (P<0.05). In conclusion, D2-40 lymphatic microvessel density showed prognostic significance with positive correlation with lymphovascular invasion, pT, and metastases to lymph nodes and liver. Immunostaining with D2-40 enhances the detection of lymphatic invasion relative to H&E staining and the endothelial marker, CD31.
Similar content being viewed by others
Main
Angiogenesis has established its role in the development and progression of a variety of malignancies, playing a crucial role in the dissemination of the tumor cells.1, 2, 3, 4, 5 However, lymphatic spread of cancer cells to lymph nodes is an important early event in the metastasis of carcinomas.6, 7 Nodal metastasis is a key factor in the staging, an important prognostic indicator for outcome, and forms the basis of treatment of human cancer.8 Regional lymph node involvement has been found to be predictor of shorter disease-free and survival in colorectal carcinoma.8
Little is known regarding the mechanisms through which tumor cells gain entry into the lymphatic system. Some investigators suggested that the lymphatic vessels have a passive role with tumor cells infiltrating pre-existing peritumoral lymphatics.9 Conversely, other investigators have suggested that lymphangiogenesis (formation of new tumor-associated lymphatics) plays an active role in the metastatic spread.10, 11, 12 Previous studies have shown the potential clinical significance of intratumoral lymphatic microvessel density as a prognostic marker, correlating with the extent of tumor spread to regional lymph nodes,11, 13 although others have not demonstrated similar findings.10, 14, 15
Previous studies have been limited by the lack of specific lymphatic endothelial markers that could be used to discriminate between lymphatics and blood vessels.16 Recently, the monoclonal antibody, D2-40, which reacts with the oncofetal membrane antigen M2A identified in ovarian carcinoma cell lines and germ-cell tumors,17 was reported to be a specific marker for lymphatic endothelium in normal and neoplastic tissue.18 In a direct comparison of D2-40 and CD31 in a series of vascular tumors, D2-40 stained all lymphangiomas and none of the hemangiomas. Conversely, CD31 stained a fraction of lymphangiomas and all hemangiomas.18
In this study, we investigated tumor lymphangiogenesis, using monoclonal antibody D2-40, and vascular endothelial growth factor (VEGF) expression as predictive markers for the risk of lymph node metastasis and their relation to other prognostic parameters in colorectal cancer patients. We also compared routine hematoxylin and eosin (H&E) staining with D2-40 immunostaining for detection of lymphatic invasion.
Materials and methods
Clinical data and pathologic tissue samples were retrieved from the computer files at the Allegheny General Hospital, Department of Pathology, from January 1998 to December 2000. Institutional Review Board approved the study. In total, 90 randomly selected patients with colorectal cancer that were surgically treated with total colectomy and vascular pedicle lymph nodes resection, were included in this study. Computerized tomography (CT) scanning was performed in all cases as a part of clinical staging. Cases with liver lesions were subjected to surgical biopsy and/or fine aspiration cytology to confirm the diagnosis of metastases.
Tissues from the specimens were fixed in 10% buffered formalin, processed and stained with H&E. H&E-stained slides of all cases were reviewed to confirm the diagnosis and evaluated for the presence of lymphovascular invasion. One paraffin block with the maximum bulk of tumor and maximum depth of invasion (pT) was chosen from each case for immunohistochemical studies. All slides showed the nonneoplastic colonic tissue-carcinoma junction.
Immunohistochemistry
Sections, 4 μm-thick, of formalin-fixed paraffin-embedded tissues were cut and mounted on coated slides. The sections were deparaffinized in xylene and rehydrated in a descending ethanol series. Heat induced epitope retrieval techniques were used for antigen retrieval as follow: citrate buffer (pH 6.0) and a steamer at 120oC for 5 min for both D2-40 and CD31, and 95°C for VEGF. Sections were incubated for 10 min in 3% hydrogen peroxide to quench endogenous tissue peroxidase. The sections were immunostained for: monoclonal antibody (Clone D2-40, Signet Laboratories, Dedham, MA, USA) at a 1:50 dilution for D2-40, a mouse monoclonal antibody (Clone 1A10, Cell Marque, Hot Springs, AR, USA) for CD31 and a rabbit polyclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a 1:500 dilution for VEGF. The Ventana Immunostainer and 3-3′ diaminobenzidine detection kit (Ventana Medical Systems, Tucson, AZ, USA) were used. Appropriate positive and negative controls were run for each batch of slides.
Quantitation and Statistics
After scanning the immunostained section at low magnification ( × 40), three areas of carcinoma with the greatest number of distinctly highlighted intratumoral lymphatic/vascular foci (hot spots) were selected by two observers (RSS & YL) at the same time. The two observers then independently evaluated the slides for microvessel counting using × 400 magnification (0.17 mm2 field) without knowledge of patient status and stains used. Single immunoreactive endothelial cells, or endothelial cell clusters separate from other microvessels, were counted as a vessel. The highest number of vessels counted was recorded and used in the statistical analysis. κ-Value between the two observers was moderate (0.69). Discordant cases were recounted and consensus resolved any discrepancy of more than 10% of the microvessel count. For VEGF staining intensity was recorded as: negative (<20% staining), or positive (>20% showing strong positivity), with a moderate κ-value (0.71). Clinical data including tumor grade, pT, lymphovascular invasion, and lymph node and liver metastases were correlated with microvessel count. Correlation with clinical data were evaluated by Spearman ‘P’ correlation and multivariate analysis. Mean differences in microvessel counts were compared with the use of paired ‘t’ tests. All statistical analyses were performed with SPSS (Statistical Package for Social Sciences, SPSS Inc., Chicago, IL, USA).
Results
The clinicopathologic findings are summarized in Table 1.
Patients with Negative Lymph Nodes
The anatomic location of the resected tumors included 18 cases in right colon, three in transverse colon and nine in left and sigmoid colon. There was a significant correlation between CD31 microvessel counts and D2-40 lymphatic microvessel counts (r=0.54, P=0.002). In this group, CD31, D2-40, and VEGF did not show significant correlation with tumor size, grade, location, and pT.
Patients with Lymph Nodes Metastases
The anatomic location of the resected tumors included 17 cases in right colon and 13 cases in left and sigmoid colon. Positive vascular pedicle lymph node was identified in three cases. Paired ‘t’ test did not show a significant difference between mean values of D2-40 and CD31 counts. There was a significant correlation between D2-40 lymphatic microvessel counts and VEGF staining (r=0.59, P=0.001). In this group, CD31, D2-40, and VEGF did not show significant correlation with tumor size, grade, location, and pT.
Patients with Liver Metastases
The anatomic location of the resected tumors included 16 cases in right colon, 3 cases in transverse colon and 11 cases in left and sigmoid colon. Positive lymph nodes were identified in 22 cases including 10 cases with positive vascular pedicle lymph nodes. There was a significant correlation between D2-40 and CD31 microvessel counts (r=0.38, P=0.01). In this group, D2-40 lymphatic microvessel counts showed significant correlation with lymphovascular invasion (r=0.35, P=0.05).
Total Group (90 Patients)
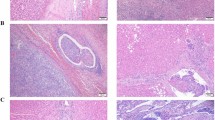
D2-40 stained the intratumor microvessels in 88/90 (98%) cases, and CD31 stained 87 (97%) cases. The mean number of lymphatic vessels identified with D2-40 was not significantly different from the mean number of microvessel identified by CD31 (18±9 and 19±8/0.17 mm2 field, respectively) (Figures 1 and 2). There was a significant correlation between microvessel counts by D2-40 and CD31 (r=0.49, P<0.05).
D2-40 expression in a colon carcinoma. (a) Note that D2-40 stains intratumoral lymphatic microvessels. Some of lymphatics are linear and collapsed. (b) D2-40 stains lymphatic vessel and predicts more lymphovascular invasion. Note small blood vessel in the left corner is negative for D2-40, indicating specificity of this marker for lymphatic vessels (IHC × 400).
Both D2-40 and CD31 microvessel counts correlated with the presence of lymphovascular invasion (r=0.34, P=0.002 and r=0.28, P=0.009, respectively), lymph node metastases (r=0.4, P<0.001 and r=0.23, P=0.05, respectively), and stage of the tumor (r=0.47, P< 0.001 and r=0.24, P=0.027, respectively). Only D2-40 lymphatic microvessel counts correlated significantly with tumor size (r=0.37, P<0.001), pT (r=0.23, P<0.05), positive vascular pedicle lymph nodes (r=0.33, P=0.002), and liver metastases (r=0.46, P<0.001). D2-40 lymphatic microvessel and CD31 microvessel counts did not show significant correlation with grade or location of the tumor. Using multivariate analysis, D2-40 lymphatic microvessel counts correlated significantly with lymphovascular invasion, lymph node, and liver metastases independent of tumor stage. D2-40 identified lymphovascular invasion in 48/90 cases, CD31 in 37/90 and routine H&E in 31/90 cases. D2-40 identified lymphovascular invasion in 17 (19%) more cases than routine H&E (Figure 2).
High VEGF expression of the neoplastic cells was identified in 61 cases (67%) and low or no expression in 29 cases (33%) (Figure 3). VEGF showed a mean value of 2.4±0.7 for colorectal cancer patients and had a significant correlation with angiolymphatic invasion (r=0.32, P=0.002), lymph node metastases (r=0.26, P=0.009), and pT (r=0.27, P=0.007). No significant correlation was found between VEGF and grade of tumor (r=0.13, P=0.12), vascular pedicle lymph nodes (r=0.16, P=0.08), and liver metastases (r=0.17, P=0.06).
Discussion
The lymphatic system is the primary pathway of metastasis for most human cancers, and the extent of lymph node involvement is a crucial prognostic factor for the patient's outcome. Recently, antibodies specific for lymphatic endothelium have become available, providing important new insights into the process of tumor-associated lymphangiogenesis and its possible clinical relevance. D2-40 has been demonstrated to recognize tumor-associated lymphatic vessels in many tumors.19, 20 In this study, we investigated the D2-40 lymphatic microvessel counts and VEGF expression as potential prognostic markers in a series of colorectal cancer patients.
Previous studies reported that solid tumors do not have lymphatic vessels,21 due to the increased interstitial pressure created by the proliferating cancer cells,22 whereas lymphatics at the tumor margin facilitate spread of tumor cells.23, 24 However, Clarijs et al,25 reported newly formed lymph vessels within the solid tumors using double staining with CD31 and PAL-E. Others have recently observed intratumoral lymphatics in melanomas,26, 27 breast,28 head and neck,29, 30 pancreatic carcinoma,31 and islet cell tumor.32 A recent study detected intratumoral lymphatics in 91% of colorectal carcinoma.33 Our results are in agreement with the later study. We found that intratumoral lymphatic vessels are present in colon cancer, using D2-40. Similar to previous studies, we found that most of intratumoral lymphatics are small and flattened,23, 32, 34 contrasting the widely open lymphatics in peritumoral regions.
Angiogenesis and lymphangiogenesis may have clinical utility in the evaluation of cancer colon, particularly for estimation of metastatic risk. Intratumoral lymphatics found to be correlated with locoregional recurrence of oral squamous cell carcinoma.30 Another study reported that intratumoral lymphatic microvessel density was higher in cervical squamous cell carcinoma cases with lymph node metastasis than in those cases without metastasis, although the difference was not significant.34 On the other hand, peritumoral lymphatic microvessel counts were approximately the same in patients with or without metastasis.34 In contrast, others proposed peritumoral lymphatic microvessel counts as a prognostic factor in other tumors.35, 36 Few available studies have addressed the presence of lymphangiogenesis in human colon carcinoma and studied its correlation with the prognostic parameters.33, 37 However, they have used different techniques and none of them used the lymphatic vessel marker D2-40. Although they observed a trend between lymphatic density and lymph node metastases, it was not statistically significant.33 Our result demonstrated that using D2-40 and CD31, microvessel counts in colorectal carcinoma correlated significantly with the presence of lymphovascular invasion and lymph nodes metastases. Moreover, lymphatic microvessel counts by D2-40 showed a statistically significant correlation with positive vascular pedicle lymph nodes and liver metastases. Using multivariate analysis, D2-40 lymphatic microvessel counts correlated significantly with angiolymphatic invasion, lymph node, and liver metastases, independent of tumor stage. The correlation between microvessel counts and metastatic disease of the colon has been reported.5, 38 These results are in accordance with angiogenesis and lymphangiogenesis being crucial factors in the metastatic process and thus for the progression of a malignant disease.
In our study, intratumoral lymphatic microvessel density was significantly correlated with microvessel density measured by CD31. Positive correlation between intratumoral lymphatic and vascular microvessel densities suggests that lymphangiogenesis and angiogenesis may occur through a common mechanism.33 Intratumoral lymph vessels are likely to play an important role in absorbing tissue fluid with metabolites excreted by tumor cells, and in maintaining an appropriate environment for tumor cells.33
Pietra et al38 and Bossi et al39 found no correlation between microvessel counts and other prognostic parameters including survival in colorectal carcinoma patients without positive lymph nodes. Our results showed similar findings in the negative lymph node patients, suggesting that neither angiogenesis nor lymphangiogenesis plays a role at this stage of colorectal carcinoma. In our study, neither microvessel counts nor VEGF overexpression showed significant correlation with any of conventional prognostic parameters. This may explain the overall good prognosis of these patients.
The contradicting results about the role of lymphangiogenesis in tumor progression may be due to differences in patient selection, methodology, and/or the types of tumors included in the analyses. Tumor lymphangiogenesis and lymphatic metastasis are complex mechanisms that can differ significantly in tumors of different types or anatomic location.30 However, the main reason for the controversial results may be due to the lack of lymphatic vessel markers. The previous investigators have used nonspecific or nonsensitive markers for their studies, such LYVE-1. Although it is claimed that LYVE-1 is a specific marker for lymphatic vessels, its value in identifying fully functional lymphatics, has been questioned. Two recent studies failed to detect lymphatics within the non-small-cell lung carcinoma using LYVE-1,40, 41 while were identified using podoplanin.40 Another study was unable to detect LYVE-1 expression in the majority of the colorectal samples, but again showed podoplanin expression.37 Therefore, LYVE-1 may not be expressed by functional lymphatics and stained nonfunctional intratumoral and peritumoral lymphatic vessels.23 Lymphatic microvessel counts by podoplanin was correlated with lymphovascular invasion in breast cancer.5
Studies have indicated that D2-40 can be useful in identifying the presence of lymphatic invasion in various malignant neoplasms.19 D2-40 outlines tumor emboli in lymphatics otherwise indiscernible by H&E, and increases the detection rate of lymphatic invasion by 16% in melanoma patients.42 The same investigators observed a trend in the association between lymphatic invasion and other prognostic indicators. Our results showed that D2-40 immunostaining is a useful immunohistochemical marker for identifying the presence of lymphatic invasion. D2-40 strongly labeled lymphatic endothelial cells, highlighting the presence of lymphatic invasion in the tumors. In our study, D2-40 increased the detection rate of angiolymphatic invasion by 19% over routine H&E. Similar to previous study,42 we showed a significant correlation between lymphatic invasion detected by D2-40, pT, and lymph node, and liver metastases.
Several reports have demonstrated that malignant cells in colorectal cancer produce a number of angiogenic growth factors, including VEGF.5, 43, 44, 45, 46, 47 Some studies have indicated that VEGF expression is an independent prognostic factor in predicting patient prognosis,5, 43, 44, 45 while others have reported no such association.46 Chung et al47 also showed that VEGF expression was significantly associated with prognosis and hematogenous spread in colorectal carcinoma. Our study showed that VEGF is a useful prognostic marker by correlating significantly with lymphovascular invasion, lymph node status and pT, although it was not an independent prognostic factor.
In conclusion, we showed that intratumoral lymphangiogenesis occurs in the colorectal carcinoma, and correlates with lymphovascular invasion, pT, lymph node, and liver metastases. In addition, D2-40 enhances the detection of lymphatic invasion relative to routine H&E staining and the panendothelial marker, CD31.
References
Folkman J . Role of angiogenesis in tumor growth and metastasis. Semin Oncol 2002;29:15–18.
Abulafia O, Triest WE, Sherer DM . Angiogenesis in malignancies of the female genital tract. Gynecol Oncol 1999;72:220–231.
Bremer GL, Tiebosch AT, van der Putten HW, et al. Tumor angiogenesis: an independent prognostic parameter in cervical cancer. Am J Obstet Gynecol 1996;174:126–131.
Saad RS, El-Gohary Y, Memari E, et al. Endoglin (CD105) and vascular endothelial growth factor as prognostic markers in esophageal adenocarcinoma. Hum Pathol 2005;36:955–961.
Saad RS, Liu YL, Nathan G, et al. Endoglin (CD105) and vascular endothelial growth factor (VEGF) as prognostic markers in colorectal cancer. Mod Pathol 2004;17:197–203.
Sleeman JP . The lymph node as a bridgehead in the metastatic dissemination of tumors. Recent Results Cancer Res 2000;157:55–81.
Stacker SA, Achen MG, Jussila L, et al. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2002;2:573–583.
Stacker SA, Baldwin ME, Achen MG . The role of tumor lymphangiogenesis in metastatic spread. FASEB J 2002;16:922–934.
Karpanen T, Alitalo K . Lymphatic vessels as targets of tumor therapy? J Exp Med 2001;194:F37–F42.
Beasley NJ, Prevo R, Banerji S, et al. Intratumoral lymphangiogenesis and lymph node metastases in head and neck cancer. Cancer Res 2002;62:1315–1320.
Maula SM, Luukkaa M, Grenman R, et al. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res 2003;63:1920–1926.
Hall FT, Freeman JL, Asa SL, et al. Intratumoral lymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg 2003;129:716–719.
Straume O, Jackson DG, Akslen LA . Independent prognostic impact of lymphatic vessel density and presence of low grade lymphangiogenesis in cutaneous melanoma. Clin Cancer Res 2003;9:250–256.
Dadras SS, Paul T, Bertoncini J, et al. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol 2003;162:1951–1960.
Schoppmann SF, Birner P, Studer P, et al. Lymphatic microvessel density and lymphovascular invasion assessed by anti-podoplanin immunostaining in human breast cancer. Anticancer Res 2001;21:2351–2355.
Choi WW, Lewis MM, Lawson D, et al. Angiogenic and lymphangiogenic microvessel density in breast carcinoma: Correlation with clinicopathologic parameters and VEGF-family gene expression. Mod Pathol 2005;18:143–152.
Marks A, Sutherland DR, Bailey D, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed in testicular germ cell tumors. Br J Cancer 1999;80:569–578.
Kahn HJ, Bailey D, Marks A . Monoclonal antibody, D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 2002;15:434–440.
Kahn HJ, Marks A . A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest 2002;82:1255–1257.
Fogt F, Zimmerman RL, Ross HM, et al. Identification of lymphatic vessels in malignant, adenomatous and normal colonic mucosa using the novel immunostain D2-40. Oncol Rep 2004;11:47–50.
Kawaguich T . Fundamental Aspect of Cancer Metastasis. Kanehara A & Co., Ltd: Tokyo, Japan, 2002.
Pepper MS . Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res 2001;7:462–468.
Padera TP, Kadambi A, Di Thomaso E, et al. Lymphatic metastasis in the absence of functional intratumoral lymphatics. Science 2002;296:1883–1886.
Van Trappen PO, Pepper MS . Lymphatic dissemination of tumor cells and the formation of micrometastases. Lancet Oncol 2002;3:44–52.
Clarijs R, Ruiter DJ, De Waal RM . Lymphangiogenesis in malignant tumors: Does it occur? J Pathol 2001;193:143–146.
Kato S . Histochemical localization of 5′-nucleotidase in the lymphatic endothelium. Acta Histochem Cytpochem 1990;23:613–620.
Kato S, Yasunaga A, Uchida U . Enzyme-histochemical method for identification of lymphatic capillaries. Lymphology 1991;24:125–129.
Bono P, Wasenius VM, Heikkila P, et al. High LYVE-1 positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res 2004;10:7144–7149.
Framchi A, Gallo O, Massi D, et al. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: a morphometeric study with clinical correlation. Cancer 2004;101:973–978.
Munoz-Guerra MF, Marazuela EG, Martin-Villar E, et al. Prognostic significance of intratumoral lymphangiogenesis in squamous cell carcinoma of the oral cavity. Cancer 2004;100:553–560.
Kurahara H, Takao S, Maemura K, et al. Impact of vascular endothelial growth factor-C and –D expression in human pancreatic cancer: its relationship to lymph node metastasis. Clin Cancer Res 2004;10:8413–8420.
Sipos B, Klapper W, Kruse ML, et al. Expression of lymphangiogenic factors and evidence of intratumoral lymphangiogenesis in pancreatic endocrine tumors. Am J Pathol 2004;165:1187–1197.
Kuroyama S, Kobayashi N, Ohbu M, et al. Enzyme histochemical analysis of lymphatic vessels in colon carcinoma: Occurrence of lymphangiogenesis within the tumor. Hepatogastroenterology 2005;52:1057–1061.
Gombos Z, Xu X, Chu CS, et al. Peritumoral lymphatic vessel density and vascular endothelial growth factor C expression in early stage squamous cell carcinoma of the uterine cervix. Clin Cancer Res 2005;11:8364–8371.
Williams CS, Leek RD, Robson AM, et al. Absence of lymphangiogenesis and intratumoral lymph vessels human metastatic breast cancer. J Pathol 2003;200:195–206.
Birner P, Schindl M, Obermair A, et al. Lymphatic microvessel density as a novel prognostic factor in early stage invasive cervical cencer. Int J Cancer 2001;95:29–33.
Parr C, Jiang WG . Quanitative analysis of lymphangiogenic markers in human colorectal cancer. Int J Oncol 2003;23:533–539.
Pietra N, Sarli L, Caruana P, et al. Is tumor angiogenesis a prognostic factor in patients with colorectal cancer and no involved nodes? Eur J Surg 2000;166:552–556.
Bossi P, Viale G, Lee AK, et al. Angiogenesis in colorectal tumors: microvessel quanitation in adenomas and carcinomas with clinicopathological correlations. Cancer Res 1995;55:5049–5053.
Renyi-Vamos F, Tovari J, Fillinger J, et al. Lymphangiogenesis correlates with lymph node metastasis, prognosis and angiogenic phenotype in human non-small cell lung cancer. Clin Cancer Res 2005;11:7344–7353.
Koukourakis MI, Giatromanolaki A, Sivridis E, et al. LYVE-1 immunohistochemical assessment of lymphangiogenesis in endometrial and lung cancer. J Clin Pathol 2005;58:202–206.
Niakosari F, Kahn HJ, Marks A, et al. Detection of lymphatic invasion in primary melanoma with monoclonal antibody D2-40: a new selective immunohistochemical marker of lymphatic endothelium. Arch Dermatol 2005;141:440–444.
Kang SM, Maeda K, Onoda N, et al. Combined analysis of p53 and vascular endothelial growth factor expression in colorectal carcinoma for determination of tumor vascularity and liver metastasis. Int J Cancer 1997;74:502–507.
Lee JC, Chow NH, Wang ST, et al. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer 2000;36:748–753.
Ishigami SI, Arii S, Furutani M, et al. Predictive value of vascular endothelial growth factor (VEGF) for metastasis and prognosis of human colorectal cancer. Br J Cancer 1998;78:1379–1384.
Takahashi Y, Tucker SL, Kitadai Y, et al. Vessel counts and expression of vascular endothelial growth factors as prognostic factors in node-negative colon cancer. Arch Surg 1997;132:541–546.
Chung YS, Maeda K, Sowa M . Prognostic value of angiogenesis in gastrointestinal tumors. Eur J Cancer 1996;32A:2501–2505.
Acknowledgements
This work was accepted as a poster presentation, in part, at the 2006 annual meeting of the United States–Canadian Academy of Pathology, Atlanta, GA, USA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saad, R., Kordunsky, L., Liu, Y. et al. Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol 19, 1317–1323 (2006). https://doi.org/10.1038/modpathol.3800651
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800651
Keywords
This article is cited by
-
Evaluation of Lymphangiogenesis in Breast Carcinomas Using D2-40 Immunostain
Indian Journal of Gynecologic Oncology (2024)
-
Multi-omics Analysis Classifies Colorectal Cancer into Distinct Methylated Immunogenic and Angiogenic Subtypes Based on Anatomical Laterality
Indian Journal of Surgical Oncology (2023)
-
Intratumoral and peritumoral lymphatic vessel density both correlate with lymph node metastasis in breast cancer
Scientific Reports (2017)
-
A potential small-molecule synthetic antilymphangiogenic agent norcantharidin inhibits tumor growth and lymphangiogenesis of human colonic adenocarcinomas through blocking VEGF-A,-C,-D/VEGFR-2,-3 “multi-points priming” mechanisms in vitro and in vivo
BMC Cancer (2015)
-
Macrophage subtype predicts lymph node metastasis in oesophageal adenocarcinoma and promotes cancer cell invasion in vitro
British Journal of Cancer (2015)