Abstract
The retromer is a protein complex that mediates retrograde transport of transmembrane cargoes from endosomes to the trans-Golgi network (TGN). It is comprised of a cargo-selection subcomplex of Vps26, Vps29 and Vps35 and a membrane-binding coat subcomplex of sorting nexins (SNXs). Previous studies identified SNX1/2 as one of the components of the SNX subcomplex, and SNX5/6 as candidates for the second SNX. How the retromer-associated cargoes are recognized and transported by molecular motors are largely unknown. In this study, we found that one of SNX1/2's dimerization partners, SNX6, interacts with the p150Glued subunit of the dynein/dynactin motor complex. We present evidence that SNX6 is a component of the retromer, and that recruitment of the motor complex to the membrane-associated retromer requires the SNX6-p150Glued interaction. Disruption of the SNX6-p150Glued interaction causes failure in formation and detachment of the tubulovesicular sorting structures from endosomes and results in block of CI-MPR retrieval from endosomes to the TGN. These observations indicate that in addition to SNX1/2, SNX6 in association with the dynein/dynactin complex drives the formation and movement of tubular retrograde intermediates.
Similar content being viewed by others
Introduction
The machinery that mediates organellar movement comprises cytoskeletal tracks and motor proteins. Among these motors are kinesins and dyneins, which are plus end- and minus end-directed microtubule motors, respectively. Dynactin was first identified as an activator of cytoplasmic dynein, and has since been found to link dynein to cargoes 1 and enhance its processivity in long-range transport 2. The dynein/dynactin motor protein complex is essential for many cellular processes, such as intracellular trafficking, mitosis and nuclear translocation 3. In the mammalian genome, the kinesin superfamily has approximately 45 members, which recognize their specific cargoes through interactions between their light chains and the divergent tail domains of heavy chains 4. How the larger and more complex cytoplasmic dynein achieves cargo specificity and regulation remains largely unknown. Cytoplasmic dynein is composed of two identical heavy chains and several associated subunits: the intermediate chains, the light intermediate chains and the light chains 5. Dynactin contains 11 subunits, including p150Glued, p50 (also known as dynamitin) and Arp1 1. Previous studies have revealed that the dynein/dynactin motor complex recognizes its cargoes through interactions between its subunits and adaptor proteins. For example, the Golgi protein Bicaudal-D binds to both dynein and the p50/dynamitin subunit of dynactin to regulate COP1-independent Golgi-ER transport 6, 7, 8. Another Golgi-associated protein, βIII spectrin, binds to the Arp1 subunit of dynactin and is believed to link the motor to membranous cargo 9. The huntingtin protein facilitates vesicular transport of brain-derived neurotrophic factor via the interaction between huntingtin-associated protein-1 and p150Glued 10, 11. Most recently, it was reported that the Rab7 effector RILP also binds to p150Glued and regulates transport of late endosomes by recruiting the dynein motor to vesicular membranes 12, 13.
Sorting nexins (SNXs) are evolutionarily conserved proteins involved in membrane trafficking and protein sorting. In humans, 33 SNX family members have been identified and they all contain a phosphoinositide (PI)-binding PX (phox homology) domain 14. In addition, some of the SNXs also have a BAR (Bin/amphiphysin/Rvs) domain, a dimerization and membrane-binding module capable of sensing membrane curvatures 14. SNXs have diverse functions and are involved in a wide variety of physiological processes. SNX1 and SNX2 are essential for sorting and trafficking of transmembrane cargoes from endosomes to the trans-Golgi network (TGN) 15. SNX3 binds to PI(3)P 16 and is required for biogenesis of endocytic carrier vesicles/multivesicular bodies 17. SNX4 associates with dynein/dynactin through its interaction with the dynein light chain 1-binding protein KIBRA and is required for transport of the transferrin receptor between peripheral early endosomes and the juxtanuclear endocytic recycling compartment 18. SNX9, in contrast, senses phosphatidylinositol 4,5-bisphosphate signals at the plasma membrane and couples actin dynamics with membrane remodeling during clathrin-mediated endocytosis, dorsal ruffle formation and clathrin-independent fluid phase endocytosis 19.
The retromer is a multimeric complex that associates with the cytosolic face of endosomal membranes and mediates retrograde transport of transmembrane cargoes from endosomes to the TGN. Among its various cargoes are the Wnt chaperone Wntless, the auxin efflux carrier PIN in plants, and mannose-6-phosphate receptors (MPR) that are sorting receptors for acid hydrolases 20, 21. The retromer consists of two independently assembled subcomplexes, the cargo selective Vps26/29/35 trimer and the membrane-binding SNX dimer 15, 22. In yeast the latter subcomplex is formed by Vps5p and Vps17p 23, 24, while in mammals the exact subunit composition of the SNX dimer is less clear. SNX1 and SNX2 are mammalian orthologs of Vps5p 15, 25, whereas there is no obvious sequence homolog of Vps17p in mammals. Most recently, an RNAi loss-of-function screen in HeLa cells identified SNX5 and SNX6 as putative components of the SNX subcomplex 26.
In vivo, retromers are present on tubulovesicular structures emanating from early endosomes and extending along microtubules 22, 27, 28, likely transport intermediates from endosomes to TGN. In vitro and in vivo studies have established that SNX1's PX domain binds to the endosome-enriched PI(3)P, and that its BAR domain enables the protein to bind curved membranes and drive membrane tubulation 27. It is believed that SNX1 and SNX2 are interchangeable subunits of the SNX subcomplex of retromer 15, and that, through coincidence sensing of the high-curvature, PI(3)P-enriched microdomains of endosomal membranes by their PX and BAR domains, the SNXs, in the form of dimer or oligomer, generate and stabilize tubular structures from vacuolar portions of endosomes. These structures are thought to allow geometric sorting of transmembrane cargoes into tubulovesicular transport intermediates destined for TGN 27.
Although the function of the retromer in endosome-to-TGN transport is well established, how the retromer-associated membranous cargoes are recognized by molecular motors is unclear. In the present study, we found that one of the interaction partners of the carboxyl (C)-terminal tail of dynactin p150Glued is SNX6. We present evidence that SNX6 binds to p150Glued, and that interaction of SNX6 with SNX1 facilitates binding of p150Glued to the retromer. We also show that a SNX6 protein with a point mutation in its PX domain loses the ability to localize to membranes and cannot mediate CI-MPR retrieval. Moreover, disruption of the SNX6-p150Glued interaction results in failure of fission/detachment of retromer-associated tubulovesicular structures from endosomes, and blocks the retrieval of CI-MPR from endosomes to the TGN, resulting in its mislocalization on peripheral endosomes. We propose that this transient interaction between a molecular motor and a sorting factor enables coupling of membrane sorting with retrograde transport from endosomes to the TGN.
Results
p150Glued interacts with SNX6, a potential subunit of the retromer protein complex
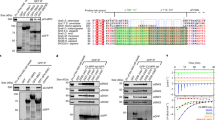
Based on previous findings that some membrane proteins bind to the p150Glued subunit of dynactin to mediate vesicular transport 10, 11, 13, 31, we reasoned that the C-terminal tail of p150Glued might serve as a binding platform for various adaptor proteins. In search of cargo adaptors for the dynein/dynactin motor, we performed a yeast two-hybrid screen of a human fetal brain cDNA library using the C-terminus of human p150Glued (amino acid residues (aa) 718-1 278) as bait. Two out of 20 positive clones were found to encode the N-terminal region of SNX6, one encompassing aa 1-136 and another 1-192. To verify this interaction, we performed additional yeast two-hybrid assays with a series of truncations and found that the shortest region of SNX6 that interacted with the C-terminus of p150Glued is aa 71-140 (Figure 1A and Supplementary information, Table S1). The shortest region of mouse p150Glued that interacted with SNX6 was the extreme C-terminus (aa 1 061-1 282) (Figure 1B and Supplementary information, Table S2). To verify the protein-protein interaction in mammalian cells, we transiently co-transfected HEK293 cells with plasmids encoding myc-tagged full-length p150Glued and Flag-tagged SNX6, and performed co-immunoprecipitation (Co-IP) with immobilized anti-Flag antibodies. We observed that myc-tagged p150Glued was co-immunoprecipitated with Flag-tagged SNX6 (Figure 1C), indicating that the interaction between SNX6 and p150Glued also occurs in cells. In contrast, p150Glued signals were barely detected in immunoprecipitates of SNX1, 2 or 5 (Figure 1C), indicating that among the four SNXs we tested, SNX6 is the primary interaction partner for p150Glued. We also verified the interaction site of p150Glued for SNX6 with a series of truncation mutants in cultured cells. Although the extreme C-terminus of p150Glued was not expressed, Co-IP of HEK293 cell extracts co-expressing SNX6 and other p150Glued fragments showed that the C-terminal fragment of aa 911-1 282 interacts strongly with SNX6 (Figure 1D). In contrast, removal of the extreme C-terminus (aa 1 061-1 282) completely abolished the binding of p150Glued to SNX6 (Figure 1D). These data together indicate that the SNX6-binding site on p150Glued lies in the extreme C-terminus (aa 1 061-1 282). These results prompted us to hypothesize that the dynein/dynactin motor complex is involved in SNX6-mediated cargo sorting and retrograde transport.
SNX6 interacts with dynactin p150Glued. (A) Yeast two-hybrid assay showing the interaction of full-length SNX6 with p150Glued C-terminus. Interaction of the fusion proteins was detected by growth of co-transformed yeast cells in the absence of adenine and histidine (-Ade, His) and the α-Galactosidase colorimetric assay as described in Materials and Methods. (B) Mapping of p150Glued's SNX6 interaction domain. (C) Co-IP from HEK293 cells transfected with constructs encoding Myc-tagged p150Glued and Flag-tagged SNXs. Cell lysates were incubated with immobilized Flag antibody. Input and bound proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to c-Myc and Flag. (D) Co-IP from HEK293 cells overexpressing Flag-tagged SNX6 and EGFP-p150Glued-N (aa 1-1 060) or C (aa 911-1 282). Cell lysates were incubated with the immobilized Flag antibody. Input and bound proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to Flag and EGFP. EGFP-SNX1 serves as positive control. Bands of EGFP-SNX1 and EGFP-p150Glued-C co-immunoprecipitated by Flag-SNX6 are indicated by asterisks.
SNX6 interacts with SNX1/2 and associates with retromer-positive endosomes
Previously it was reported that SNX6 is a potential component of the mammalian retromer, a multimeric protein complex that mediates retrograde transport of membranous cargoes from endosomes to TGN. To determine whether SNX6 is indeed a component of retromer, we performed Co-IP assays and detected SNX6 in immunoprecipitates of SNX1 and SNX2 (Figure 2A). No interaction between SNX5 and SNX6 was detected in Co-IP (Figure 2A), indicating that SNX6 primarily interacts with SNX1/2. Moreover, SNX5 was detected in immunoprecipitates of SNX1 and SNX2 (Figure 2B), indicating that both SNX5 and SNX6 interact with SNX1/2. In sedimentation analyses of HeLa cell lysates on linear sucrose gradients, consistent with previous studies 15, SNX1, 2, 5 and 6 co-sedimented in lighter fractions, whereas Vps35 migrated in heavier fractions (Figure 2C), suggesting that SNX6 and SNX1/2/5 form a subcomplex. Moreover, the peak fractions of the dynein/dynactin complex were distinct from those of the retromer subunits (Figure 2C), suggesting that the motor-retromer association is weak or transient in vivo.
SNX6 binds to SNX1/2 and localizes to retromer-associated endosomal membrane. (A) Co-IP of SNX6 and SNX1, 2 and 5. HEK293 cells were co-transfected with constructs encoding HA-tagged SNX6 and Flag-tagged SNX1, SNX2, or SNX5. NP40-solubilized extracts were used for immunoprecipitation with immobilized Flag antibodies. Immunoblot was probed using antibodies to HA and Flag. (B) As in (A), but cells were transfected with constructs encoding myc-tagged SNX5 and Flag-tagged SNX1 or SNX2. The immunoblot was probed using antibodies to c-Myc and Flag. (C) Sedimentation analysis of HeLa cell lysates on 5%-25% sucrose gradients. Samples were analyzed by SDS-PAGE and immunoblotting with antibodies to p150Glued, dynein intermediate chain (DIC), SNX1, SNX2, SNX5, SNX6 and Vps35. (D) Quantification of SNX6's colocalization with organelle markers. HeLa cells were fixed and immunostained with antibodies to SNX6 and SNX1, EEA1, Syntaxin 8, TGN46, or Syntaxin 6. Data represent means ± SEM (n = 9-12) (**P < 0.05; ***P < 0.001). Values of Mander's overlap were analyzed using one-way ANOVA with a Tukey test.
To define subcellular localization of SNX6, we performed co-immunofluorescence staining assays on HeLa cells using various vesicular markers (Supplementary information, Figure S1). Quantitative analyses of confocal images revealed that SNX6 primarily co-localized with SNX1 and EEA1 to punctate cytoplasmic foci, with little colocalization with the late endosome marker Syntaxin 8 or the trans-Golgi marker TGN46 (Figure 2D), indicating that SNX6 primarily localizes to retromer-associated endosomal membranes.
To test whether SNX6 resides on the same membrane domains as does SNX1, we performed time-lapse fluorescence microscopy of HeLa cells co-expressing low levels of transiently transfected EGFP-SNX6 and mCherry-SNX1. Consistent with previous reports 26, we observed colocalization of SNX6 with SNX1-labeled vesicles and tubulovesicular structures (Supplementary information, Movie S1). To confirm that SNX6 localizes to early endosomes, we also performed live imaging of HeLa cells co-expressing YFP-EEA1 and mCherry-SNX6. Similar to SNX1 and SNX6 co-expression experiments, we visualized numerous EEA1-labeled endosomal vesicles that were also SNX6-positive (Supplementary information, Movie S2). These data together indicate that SNX6 localizes to retromer-associated endosomal membranes in vivo.
SNX6's recruitment to endosomal membranes requires PI(3)P and SNX1/2 interaction
Since the major PI for PX domain binding is PI(3)P, and the primary intracellular location of SNX6 is the PI(3)P-rich endosomal membrane, we reasoned that the recruitment of SNX6 to endosomal membranes might be mediated by binding of PI(3)P through its PX domain (aa 20-169). To determine whether SNX6 binds to PI(3)P-positive compartments, we transfected HeLa cells with a plasmid encoding mCherry-FYVEEEA1, a marker of PI(3)P domains 30, 32, 33, and examined the subcellular distribution of endogenous SNX6 by fluorescence microscopy. SNX6 partially co-localized with mCherry-FYVEEEA1-labeled vesicles (Supplementary information, Figure S2) in cells overexpressing mCherry-FYVEEEA1, consistent with SNX6 binding to PI(3)P in vivo. To test whether SNX6's membrane association is PI(3)P dependent, we treated cells with the PI3K inhibitor wortmannin (WM) to deplete membrane PI(3)P, followed by immunofluorescence staining to examine the distribution of SNX6 34, 35. Upon addition of WM, similar to SNX1/2, endogenous SNX6 was dispersed (Figure 3A and Supplementary information, Figure S3), indicating that its endosomal localization is dependent on PI(3)P.
Recruitment of SNX6 to endosomal membranes requires PI(3)P and SNX1/2. (A) HeLa cells were treated with DMSO (upper panels) or 200 nm wortmannin (lower panels) for 20 min, fixed and immunostained for SNX1 and SNX6. (B) HeLa cells were transfected with a control non-targeting siRNA, or siRNAs to SNX1 and SNX2 and analyzed by immunostaining. A cell depleted of both SNX1 and SNX2 is outlined in white. (C) HeLa cells were transfected with control siRNA (a-d, i-l) or siRNA to SNX6 (e-h, m-p), fixed and immunostained with antibodies to SNX6 and SNX1 (a-h) or SNX2 (i-p), followed by the appropriate secondary antibodies. (D) HEK293 cells were co-transfected with plasmids encoding HA-tagged SNX1 and Flag-tagged SNX2, SNX5 or SNX6. Cells were lysed for Co-IP with immobilized Flag antibody after 20 min of wortmannin treatment. Input and bound proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to HA and Flag. Bar, 10 μm.
Since depletion of PI(3)P also leads to SNX1/2's dissociation from endosomal membranes 22, and SNX6 interacts with SNX1/2, it is likely that SNX6's membrane association requires endosomal SNX1/2. To test whether SNX6's recruitment to membranes is SNX1/2-dependent, we depleted SNX1/2 from HeLa cells by RNAi. Immunofluorescence microscopy showed that this depletion caused loss of punctate staining of SNX6 (Figure 3B), indicating SNX6's dissociation from endosomes. Moreover, SNX6 signals in SNX1/2-depleted cells were much weaker than control cells (Figure 3B). Conversely, to determine whether SNX1/2's recruitment to membranes requires SNX6, we depleted SNX6 from HeLa cells by RNAi. Depletion of SNX6 did not have any effect on the membrane localization of SNX2, indicating that SNX2's association with membranes does not require SNX6 (Figure 3C, m-p). In contrast, consistent with previous reports 26, very weak if any SNX1 signals were detected in SNX6-depleted cells (Figure 3C, e-h), suggesting that SNX1 might be stabilized via complexing with SNX6. Moreover, although depletion of PI(3)P caused dissociation of SNX1, SNX2 and SNX6 from the membrane, it did not weaken SNX1's interaction with SNX5 or SNX6 (Figure 3D), indicating that the interactions between SNXs do not require their membrane association.
Role of SNX6's PX domain in membrane targeting and protein-protein interactions
The N-terminus of SNX6 is capable of binding to p150Glued in addition to its predicted affinity for PI(3)P. To further investigate its function(s), we created a series of point mutations in the PX domain (Figure 4A) to mutate residues predicted to be important for PI(3)P-binding based on the PX domain structures of SNX1 and p40phox 36, 37 and tested the membrane association of the mutants by immunofluorescence microscopy. The R68A mutant, with the arginine residue replaced with alanine, was diffusely distributed in both cytosol and nucleus when overexpressed (Supplementary information, Figure S4), indicating that its binding to the membrane was disrupted. In contrast, the Q69A and R97A mutants remained on vesicular structures and co-localized with SNX1, as shown by immunofluorescence staining (Supplementary information, Figure S4), indicating that these amino acid substitutions did not affect membrane localization.
A point mutation in SNX6's PX domain abolishes its membrane localization. (A) Sequence alignment of the PX domains of human SNX1, 2, 5 and 6. Identical amino acid residues are shaded in black and similar residues are shaded in gray. Asterisks indicate residues predicted to be critical for SNX6's PI-binding. (B) Co-IP of HEK293 cells overexpressing Flag-tagged SNX6 WT or mutants with HA-tagged SNX1. Cell lysates were incubated with immobilized Flag antibody. The left panel shows immunoblot probed with antibodies to Flag and HA. The right panel shows quantification of protein-protein interactions using the ImageJ software (NIH). (C) Co-IP of HEK293 cells overexpressing Flag-tagged SNX6 WT or mutants with myc-tagged p150Glued. Cell lysates were incubated with immobilized Flag antibody. The left panel shows immunoblot probed using antibodies to Flag and c-Myc. The right panel shows quantification of protein-protein interactions.
To further investigate whether SNX6 membrane localization is required for its interaction with SNX1 and p150Glued, we performed Co-IP assays using cells co-expressing SNX1 or p150Glued and the mutants. Quantitative analysis of immunoblotting results showed that the interaction of R68A with SNX1 was weaker than wild type (WT), whereas SNX1 binding of other mutants was slightly increased (Figure 4B). Similarly, the interaction of R68A with SNX2 was weaker than WT as detected by Co-IP assay (data not shown). Interactions between p150Glued and all point mutants were enhanced (Figure 4C). These results suggest that SNX6 membrane association, which depends on its interaction with SNX1/2, is not required for its interaction with p150Glued.
CI-MPR trafficking from endosome to TGN requires the full activity of SNX6
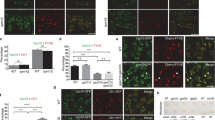
Since the motor complex needs to be tethered to its cargo by adaptor proteins to exert its function, we reasoned that if SNX6 functions as an adaptor for the motor, once it dissociates from membranous cargo, the link between motor and cargo would be severed and vesicular transport would be disrupted. To test whether the cytosolic SNX6 R68A mutant disrupts retromer-mediated transport, we examined CI-MPR distribution in HeLa cells overexpressing SNX6 R68A. Confocal immunofluorescence microscopy showed that CI-MPR distribution was unchanged upon SNX6 R68A mutant expression (Figure 5A, d-f), possibly because the cytosolic mutant's affinity for SNX1 is much lower than that of WT SNX6 (Figure 4B), which is abundant in cells. Since SNX6 binds to SNX1, and depletion of both proteins causes CI-MPR dispersal 26, we reasoned that suppressing protein levels of both SNX1 and SNX6 would decrease the intracellular concentrations of these retromer components to such a low level that they become limiting factors for the transport machinery required for CI-MPR retrieval (Supplementary information, Figure S5). To examine SNX6's role in endosome-to-TGN transport, we depleted SNX1/6 (Supplementary information, Figure S5) in HeLa cells by RNAi and attempted to rescue the disruption of CI-MPR retrieval by WT or mutant SNX6 proteins expressed at low levels. Confocal microscopy showed that in HeLa cells depleted of both SNX1 and SNX6, block of CI-MPR retrieval was completely rescued by both the Flag-tagged SNX6 WT and Q69A mutant (Figure 5B, a-c and g-i). In contrast, CI-MPR remained dispersed in cells expressing Flag-tagged SNX6 R68A mutant (Figure 5B, d-f), indicating that a membrane-bound SNX6 is required to mediate retrograde transport of retromer-associated vesicular cargoes.
CI-MPR retrieval requires membrane-associated SNX6. (A) HeLa cells growing in a 24-well plate were transfected with plasmids encoding Flag-tagged wild type, R68A, or Q69A (500 ng each), fixed and immunostained with antibodies to Flag and CI-MPR. (B) HeLa cells co-transfected with siRNA duplexes targeting SNX1 and SNX6 (20 nM each) for 24 h were co-transfected with plasmids encoding RNAi-resistant, Flag-tagged SNX6 WT, R68A or Q69A (75 ng each) and siRNA duplexes targeting SNX1 and SNX6 (10 nM each). Cells were fixed 48 h after the second round of transfection and immunostained with antibodies to SNX1, Flag and CI-MPR. SNX1-depleted cells overexpressing Flag-tagged SNX6 are indicated by asterisks. Bar, 10 μm.
Retrieval of CI-MPR from endosomes to the TGN requires dynein/dynactin motor activity and the microtubule cytoskeleton
Most of the retrograde vesicular transport from the cell periphery to the center requires activity of the microtubule-based dynein/dynactin motor complex. To determine whether the retromer-mediated retrieval of CI-MPR from endosomes to the TGN is dynein/dynactin-driven and microtubule-dependent, we examined CI-MPR distribution in HeLa cells disrupted of either the microtubule network or dynein/dynactin function. Incubation of cells with nocodazole induced redistribution of CI-MPR from the TGN to peripheral puncta (Figure 6A, e-h). Overexpression of a Flag-tagged p50/dynamitin, which disassembles the dynein/dynactin protein complex 38, 39, caused a similar phenotype (Figure 6B, a-d), indicating that both the intactness of the microtubule network and motor activity are required for CI-MPR retrieval.
Subcellular distribution of CI-MPR requires an intact microtubule network and the activity of dynein/dynactin motor complex. (A) HeLa cells were treated with DMSO (a-d) or nocodazole (20 μM in DMSO, e-h) for 3 h, fixed and immunostained with antibodies to α-tubulin and CI-MPR. (B) HeLa cells transfected with plasmids encoding Flag-tagged p50/dynamitin (a-d), p150Glued-N (aa 1-1 060, e-h), p150Glued-C (aa 911-1 282, i-l) or MAP1B light chain (m-p) were fixed 24 h later and immunostained with antibodies to Flag and CI-MPR. Flag-MAP1B-LC 41 served as negative control. Bar, 10 μm.
Since p150Glued binds to SNX6 through its C-terminus, and its N-terminus mediates dynein interaction, we reasoned that in the absence of the cargo-recognition site, the amino (N)-terminus alone could serve as a dominant negative mutant to compete with the full-length protein in the motor complex. As expected, overexpression of the N-terminal region of p150Glued (aa 1-1 060) also perturbed CI-MPR distribution, causing it to redistribute from TGN to peripheral endosomes (Figure 6B, e-h), whereas overexpression of its SNX6-binding C-terminus (aa 911-1 282) had no obvious effect (Figure 6B, i-l), probably because the C-terminus cannot assemble into the dynactin complex by itself and thus cannot compete with the endogenous, membrane-associated WT protein for vesicular cargoes 12. Therefore, these data establish that CI-MPR's retrieval from endosomes to the TGN is both microtubule- and dynein/dynactin-dependent.
The dynein/dynactin motor complex is recruited to transport vesicles through its interaction with the retromer complex
For the motor protein to move its vesicular cargoes, it needs to recognize the cargo and dock onto the membrane surface. Having established that SNX6 is part of the retromer complex and that dynein/dynactin is required for retromer-mediated transport from endosomes to the TGN, we reasoned that the recruitment of the dynein/dynactin motor complex to retromer-associated vesicles might require SNX6 function. If this is the case, then we should be able to detect an indirect interaction between components of the retromer other than SNX6 and the motor complex. Indeed, in the presence of SNX6, we detected p150Glued in SNX1/2 immunoprecipitates of co-transfected HEK293 cells (Figure 7A), indicating that the interaction between p150Glued and SNX1 or SNX2 is mediated by SNX6.
SNX6 recruits dynein/dynactin to the retromer. (A) HEK293 cells were co-transfected with plasmids encoding myc-p150Glued and Flag-SNX1 or Flag-SNX2, with or without plasmid encoding HA-SNX6. Cell lysates were incubated with immobilized Flag antibody. Input and bound proteins were analyzed by SDS-PAGE and immunoblotting with antibodies to c-Myc, Flag and HA. (B) Co-IP of endogenous p150Glued and DIC from HeLa cell lysates with antibodies to SNXs. Immunoblot was probed with antibodies to p150Glued, DIC, SNX5 and SNX6.
To verify that the motor protein complex is recruited to the retromer through SNX6-p150Glued interaction in vivo, we performed endogenous Co-IP from HeLa cell lysates to detect DIC together with p150Glued in SNX6 immunoprecipitates. Our attempts to detect endogenous p150Glued and DIC from SNX6 immunoprecipitates failed repeatedly (Figure 7 and data not shown), probably because the interaction between SNX6 and the motor is weak and/or transient. To further verify the motor-retromer interaction, we performed endogenous Co-IP from HeLa cell lysates with a mixture of SNX antibodies. We detected weak signals of p150Glued and DIC in the immunoprecipitates of SNX1/2/6 (Figure 7B), and stronger signals in immunoprecipitates of both SNX5/6 and SNX1/2/5/6 (Figure 7B), suggesting that formation of a SNX subcomplex enhances SNX6's interaction with p150Glued and the rest of the motor complex.
Fission/detachment of transport intermediates from endosomal membranes requires the longitudinal force generated by the dynein/dynactin motor
To assess the role(s) of dynein/dynactin in the dynamics of retromer-associated endosomes in live cells, we performed time-lapse fluorescence microscopy of HeLa cells co-transfected with plasmids encoding mCherry-SNX1 and EGFP-p150Glued-N (aa 1-1 060) or EGFP-p150Glued-C (aa 911-1 282) (Figure 8, Supplementary information, Figure S6 and Movies S3, S4, S5). Overexpression of p150Glued C-terminus had no effect on elongation and detachment of SNX1-labeled tubulovesicular structures in all of the 10 cells imaged (Figure 8A and Supplementary information, Movie S3). In contrast, in most cells (11 out of 14) overexpressing p150Glued-N, some SNX1-labeled vesicles were enlarged and very few tubular structures were detected, most likely resulting from lack of pulling forces exerted by the motor complex (Figure 8B and Supplementary information, Movie S4). In the other three cells we observed very long SNX1-labeled tubules, emanating from endosomes and then retracting (Figure 8C and Supplementary information, Movie S5), possibly resulted from membrane tubulation driven by SNX oligomers that was not sustained without the force generated by motor proteins. Moreover, these vesicles failed to detach from endosomes (Figure 8C and Supplementary information, Movie S5). Taken together, these data indicate that the SNX6-p150Glued interaction is required for the formation and exit of retromer-associated transport vesicles from endosomes to the TGN.
Fission/detachment of transport vesicles from endosomal membranes requires SNX6-mediated dynein/dynactin motor activity. HeLa cells co-transfected with plasmids encoding mCherry-SNX1 and EGFP-tagged p150Glued fragment were imaged by time-lapse fluorescence microscopy. Static images at various time points were extracted from Supplementary information, Movies S3, S4, S5 (see also Supplementary information, Figure S6). (A) A cell co-expressing mCherry-SNX1 and EGFP-p150Glued-C (aa 911-1 282). An example of detaching tubules is indicated by the white arrowhead. (B) A cell co-expressing plasmids encoding mCherry-SNX1 and EGFP-p150Glued-N (aa 1-1 060). A SNX1-positive vesicle is indicated by the white arrowhead. (C) Same as in (B). A SNX1-positive tubule is indicated by the white arrowhead. Time after the start of imaging is shown at the top left corner of each panel.
Discussion
In the present study, we have examined the role of SNX6 in retromer-mediated endosome-to-TGN transport. We have shown that SNX6 interacts with both SNX1 and SNX2 and localizes to retromer-associated endosomal membranes. The PX domain of SNX6 not only facilitates its recruitment to the endosomal membrane, but also provides the interaction site for dynactin p150Glued and recruits the motor complex to retromer-associated endosomal subdomains. In this manner, SNX6 coordinates the function of the retromer coat complex and the molecular motor to couple membrane tubulation/cargo sorting with retrograde transport.
The PX domains of SNX family members function to target proteins to their specific vesicular membranes. Although previous research identified several PX domains with high affinity for PI(3)P, the majority of PX domains are found to have a low affinity for PIs that is insufficient to target them to vesicles 20. Other factors, besides PIs in vesicular membranes, are required for proper targeting of PX domain-containing proteins 20. Our study of SNX6 mutants identified amino acid residues in the PX domain that are critical either for SNX6 membrane targeting or for its interaction with SNX1 and p150Glued, or both. These results suggest that protein folding and conformational change might contribute to the regulation of SNX6's affinity for phospholipid and interaction-protein partners, which in combination target SNX6 to proper membranes and protein complexes to exert its biological function.
One main finding of this work is the demonstration that the formation and fission of retromer-associated transport vesicles from endosomes rely on the interaction between membrane-associated SNX6 and the motor protein complex. First, a point mutation in the SNX6 PX domain greatly weakens the binding of SNX6 to SNX1 and abolishes its membrane localization. Second, this mutant fails to rescue the defect in CI-MPR recycling from endosomes to the TGN when SNX1 and SNX6 are both depleted. Finally, the biogenesis and exit of SNX1-decorated transport intermediates from endosomes are blocked when the interaction between the retrograde motor and retromer-associated cargo is disrupted.
While this paper was in preparation, the SNX6-p150Glued interaction was reported by Peter J Cullen's group 40. Consistent with our findings, they discovered that SNX6 associates with p150Glued and that SNX6 interacts with SNX1/2 40. They also demonstrated that suppression of p150Glued redistributes the retromer-labeled endosomes from regions surrounding the TGN to a more peripheral location 40. In our study, to investigate the role of adaptor protein-mediated motor-cargo interaction, we overexpressed the N-terminus of p150Glued as a dominant-negative mutant to compete with endogenous WT protein for dynein, and found that this mutant, which lacks the SNX6 interaction site, causes failure in formation, stabilization and exit of retromer-associated transport intermediates from endosomes, therefore blocking CI-MPR retrieval (Figure 8 and Supplementary information, Movies S3, S4, S5). Our results clearly indicate that membrane tubulation driven by SNX oligomerization per se is not sufficient for the formation and fission of transport vesicles from endosomes, and that retromer-mediated vesicular sorting and transport require the retrograde motor activity.
Based on our findings, we propose the following model for the mechanistic roles of SNX6 and dynein/dynactin in retromer-mediated endosome-to-TGN transport (Figure 9). At the early endosome, SNX1/2 binds to PI(3)P-enriched membranes through coincidence detection by the PX and BAR domains 27. SNX6 is recruited to the SNX1/2-associated subdomains through its binding to SNX1/2. The Vps26/29/35 cargo-selection complex is recruited to endosomes through its interaction with the SNX subcomplex and Rab7 15, 34. The membrane-associated SNX6 binds to dynactin p150Glued and recruits the motor complex to the endosomes. Oligomerization of SNX BAR domains generates and stabilizes the tubular structures from the vacuolar portions of endosomes, which are further enhanced by the pulling force generated by the dynein motor. Finally, the longitudinal force of the motor imposed on the endosomal tubules assists fission of the cargo-containing transport vesicles from endosomal membranes, which then move along microtubule tracks to the TGN, their final destination (Figure 9). In this model, SNX6 acts as a cargo adaptor for transmembrane cargoes such as CI-MPR. It not only tethers the motor protein to retromer-associated membranous cargoes, but also facilitates the formation and final detachment of the transport carriers from endosomes. Interestingly, we also observed significant colocalization of SNX6 with Syntaxin 6, a t-SNARE protein localized on the TGN (Figure 2 and Supplementary information, Figure S1), suggesting that SNX6 might be involved in cargo recognition and release at the TGN as well. More experiments will be needed to define the fate of the SNXs and other components of the retromer coat complex along the route of endosome-to-TGN transport. It also remains to be determined whether the PI-binding specificity and affinity of SNXs are regulated upon binding of protein factors such as dynactin p150Glued and tethering factors at the recipient membrane (TGN).
Materials and Methods
Antibodies and plasmids
Mouse monoclonal antibodies against SNX1, SNX2, EEA1, p150Glued, Syntaxin 6, Syntaxin 8 and rabbit polyclonal anti-LAMP1 were from BD Biosciences. Mouse monoclonal anti-DIC (74-1), and goat polyclonal antibodies against SNX1, SNX2, SNX5 and SNX6 were from Santa Cruz Biotech. Mouse monoclonal anti-CI-MPR and rabbit polyclonal anti-TGN46 were from Abcam. Rabbit polyclonal anti-CI-MPR was a kind gift from Dr Juan S Bonifacino. Mouse monoclonal antibodies against α-tubulin, β-actin, Flag (M2), HA and rabbit polyclonal anti-Flag were from Sigma-Aldrich. Goat polyclonal anti-Vps35 was from IMGENEX. Rabbit polyclonal anti-Myc and mouse monoclonal anti-GFP were from MBL. Fluorescent conjugates of secondary antibodies were purchased from Invitrogen, and horse radish peroxidase (HRP)-conjugated secondary antibodies were from Pierce.
Flag-hSNX6 and HA-hSNX6 expression constructs were kindly provided by Dr Hiroyoshi Ariga 29. Human SNX6 full-length cDNA and fragments were PCR-amplified from the Flag-hSNX6 construct, and cloned into pGADT7 (Clontech) for yeast two-hybrid analyses. The His-mSNX6 construct was generated by PCR amplification from mouse brain cDNA and cloned into pET-28a(+) vector (Novagen). EGFP- and mCherry-mSNX6 were PCR-amplified from the His-mSNX6 plasmid and cloned into pEGFP-C1 and pmCherry-C1 (Clontech), respectively, for live cell imaging. Mouse SNX1 and SNX2 constructs were obtained by PCR amplification of cDNA from plasmids provided by Dr Wei Li and mouse brain cDNA, respectively, and cloned into pGBKT7, pGADT7, pEGFP-C2, mCherry-C2, pCMV-HA (Clontech) and pCMV-Tag2B (Stratagene). Mouse SNX5 was PCR-amplified from mouse brain cDNA, and cloned into pGBKT7, pGADT7 (Clontech) and pCMV-Tag2B (Stratagene). The full-length cDNA of mouse p150Glued was PCR-amplified from mouse brain cDNA, and cloned into pCMV-Tag2A and pCMV-Tag3B (Stratagene). The p150Glued fragments were PCR-amplified from the Flag-mp150Glued plasmid, and cloned into pGADT7, pCMV-Tag2A and pEGFP-C1 (Clontech). Flag-hSNX6 R68A, Q69A, and R97A mutants were generated by site-directed mutagenesis. RNAi-resistant constructs were generated by creating point mutations in the siRNA site (primer sequences for mutagenesis: forward 5′-atg gtt aaa tca gct aat gga gta atc gt-3′, reverse 5′-att act cca tta gct gat tta acc atg tt-3′). The mCherry-FYVEEEA1 plasmid was constructed by PCR amplification of EEA1's FYVE domain (aa 1 252-1 411) 30 from HeLa cell cDNA and cloning into pmCherry-C1 (Clontech).
Yeast two-hybrid screen and assays
Yeast two-hybrid screen and assays using the MATCHMAKER Gal4 system (Clontech) were performed following the manufacturer's protocols. The C-terminus (aa719-1 278) of p150Glued was used as bait to screen a human brain cDNA library (Clontech). Briefly, protein-protein interaction was examined by growth test of yeast transformants on SD medium lacking leucine, tryptophan, adenine and histidine (SD-Leu-Trp-Ade-His). The relative strength of interactions was evaluated by comparing the color of colonies on SD-Leu-Trp-Ade-His containing 2 mg/ml X-α-Gal. The AH109 strain co-transformed with control vectors pGBKT7-53 and pTD1-1 served as positive control.
Cell culture and transfection
HeLa and HEK293 cells were maintained at 37 °C with 5% CO2 in Dulbecco's modified Eagle's medium, DMEM (Hyclone), supplemented with 10% (vol/vol) fetal bovine serum (Hyclone), 100 U/ml penicillin (Hyclone) and 100 μg/ml streptomycin (Hyclone). For transient transfection, cells were plated on glass coverslips and transfected with plasmid DNA at 40%-50% confluency using LipofectamineTM 2000 (Invitrogen) following the manufacturer's instructions. Immunostaining was performed 24 h after transfection.
For siRNA-mediated gene knockdown, siRNA duplexes were purchased from Genepharma. The siRNAs were transiently transfected using either Oligofectamine or Lipofectamine™ 2000 (Invitrogen) following the manufacturer's instructions. The final concentration of siRNA duplexes was 20 nm. SiRNA target sequences were: control: AAG ACA AGA ACC AGA ACG CCA, SNX1: AAG AAC AAG ACC AAG AGC CAC, SNX2: AAG UCC AUC AUC UCC AGA ACC (Carlton et al., 2005), SNX5: CUA CGA AGC CCG ACU UUG AUU and SNX6: UAA AUC AGC AGA UGG AGU AUU (Wassmer et al., 2007). For siRNA transfection, HeLa cells were seeded at 30%-40% confluency and transfected twice at 24-h intervals. For rescue experiments, plasmid DNA and siRNAs were cotransfected with Lipofectamine™ 2000 24 h after the first round of siRNA transfection. Cells were analyzed 48 h after the second round of transfection.
For WM treatment, cells were washed once and incubated in Minimum Essential Medium Eagle (MEM, Hyclone) containing 2.5 g/l NaHCO3, 20 mM HEPES, pH 7.4, and 0.6% (wt/vol) bovine serum albumin (MEM-BSA) at 37 °C for 30 min. Cells were then incubated with dimethyl sulfoxide (DMSO) or 200 nM WM for 20 min at 37 °C and then immediately fixed and immunostained with goat anti-SNX6 and mouse anti-SNX1, followed by the appropriate secondary antibodies.
Immunofluorescent staining and confocal microscopy
For immunofluorescence microscopy, cells were fixed with 4% (wt/vol) paraformaldehyde in PBS for 10 min at room temperature, except for p150Glued-transfected and nocodazole-treated cells, which were fixed with 100% methanol at −20 °C for 6 min. Cells were permeabilized using 0.1% (wt/vol) Triton X-100 (Sigma-Aldrich) for staining with most antibodies, except for LAMP1, Syntaxin 6 and Syntaxin 8, for which 0.05% (wt/vol) Saponin (Sigma-Aldrich) was used for permeabilization. Incubation with the primary antibodies was followed by incubation with fluorescently labeled secondary antibodies. 4′,6-Diamidino-2-phenylindole was used for nuclear staining. For triple staining of SNX1/2, SNX6 and CIMPR, HeLa cells were first incubated with goat anti-SNX6, followed by Alexa Fluor 594-conjugated donkey anti-goat IgG. Cells were then incubated with mouse anti-SNX1 or SNX2, followed by Alexa Fluor 488-conjugated goat anti-mouse IgG. Finally, cells were incubated with mouse anti-CIMPR, followed by Alexa Fluor 647-conjugated donkey anti-mouse IgG.
Confocal images were obtained using the Spectral Imaging Confocal Microscope DIGITAL ECLIPSE C1Si (Nikon, Japan) equipped with a 408-nm diode, 457/477/488/514-nm Ar, 543-nm He-Ne and 640-nm lasers. Images were acquired using a 100× Plan Apochromat VC NA 1.40 oil objective and analyzed using the NIS-Elements AR software provided by Nikon.
Subcellular fractionation
HeLa cells were lysed in ice-cold acetate buffer (0.1% [vol/vol] NP-40, 25 mM HEPES, pH 7.4, 100 mM K-acetate, 15 mM Mg-acetate, 50 mM EGTA, 1 mM DTT) supplemented with protease inhibitors and centrifuged at 1 000× g for 10 min at 4 °C to obtain a postnuclear fraction. This fraction was then centrifuged at 16 000× g for 15 min at 4 °C. One ml of the supernatant was layered on top of a linear 5%-25% (wt/vol) sucrose gradient in acetate buffer, pH 7.4. Samples were centrifuged at 100 000× g for 12 h at 4 °C. Fractions were collected from the bottom of the tube and analyzed by SDS-PAGE and immunoblotting.
Live cell imaging
HeLa cells grown to 40%–50% confluency on chambered coverglass (Nalge Nunc) were transfected with Lipofectamine™ 2000 and imaged at 37 °C with 5% CO2 24 h later. Time-lapse fluorescence images were acquired with the Spectral Imaging Confocal Microscope DIGITAL ECLIPSE C1Si equipped with a T-PFS Perfect Focus Unit and a 100× Plan Apochromat VC NA 1.40 oil objective. Images were captured with the EZ-C1 Spectral software (Nikon). Single-channel images were acquired at 2-s intervals, double-channel images were acquired at 3.5-4-s intervals. Images were exported as TIFF files and Quicktime videos were produced using NIS-element AR (Nikon).
Immunoprecipitation (IP)
HeLa or HEK293 cells at approximately 95% confluency were washed with ice-cold PBS and lysed either with lysis buffer 1 (0.05% [vol/vol] NP-40, 15 mM Tris-HCl, pH 7.4, 50 mM NaCl, 5 mM EDTA) supplemented with protease inhibitors for endogenous IP or with lysis buffer 2 (0.1% [vol/vol] NP-40, 50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA) supplemented with protease inhibitors for all other IPs. The cells were then centrifuged at 16 000 × g for 15 min at 4 °C. For Flag IP, the high-speed supernatants were incubated with anti-Flag Affinity Gel (Sigma-Aldrich) at 4 °C for 2 h; for other IPs, the antibody (1 μg) was added to the cell lysate and incubated at 4 °C for 2 h, followed by incubation with Protein A/G PLUS-agarose (Santa Cruz) pre-equilibrated in lysis buffer overnight at 4 °C. Precipitates were washed twice in lysis buffer and three times in ice-cold PBS. Immunoprecipitates were eluted from the agarose by boiling in 2× SDS Gel loading buffer (100 mM Tris-Cl pH 6.8, 4% SDS, 0.2% bromophenol blue, 20% [vol/vol] glycerol, 10% [vol/vol] 2-mercaptoethanol) and subjected to SDS-PAGE and immunoblotting. Immunoblots were imaged with an Epichemi3 Darkroom system (UVP BioImaging Systems).
( Supplementary information is linked to the online version of the paper on the Cell Research website.)
References
Schroer TA . Dynactin. Annu Rev Cell Dev Biol 2004; 20:759–779.
King SJ, Schroer TA . Dynactin increases the processivity of the cytoplasmic dynein motor. Nat Cell Biol 2000; 2:20–24.
Reinsch S, Karsenti E . Movement of nuclei along microtubules in Xenopus egg extracts. Curr Biol 1997; 7:211–214.
Duncan JE, Goldstein LS . The genetics of axonal transport and axonal transport disorders. PLoS Genet 2006; 2:e124.
Vale RD . The molecular motor toolbox for intracellular transport. Cell 2003; 112:467–480.
Hoogenraad CC, Akhmanova A, Howell SA, et al. Mammalian Golgi-associated Bicaudal-D2 functions in the dynein-dynactin pathway by interacting with these complexes. EMBO J 2001; 20:4041–4054.
Hoogenraad CC, Wulf P, Schiefermeier N, et al. Bicaudal D induces selective dynein-mediated microtubule minus end-directed transport. EMBO J 2003; 22:6004–6015.
Matanis T, Akhmanova A, Wulf P, et al. Bicaudal-D regulates COPI-independent Golgi-ER transport by recruiting the dynein-dynactin motor complex. Nat Cell Biol 2002; 4:986–992.
Holleran EA, Ligon LA, Tokito M, et al. Beta III spectrin binds to the Arp1 subunit of dynactin. J Biol Chem 2001; 276:36598–36605.
Engelender S, Sharp AH, Colomer V, et al. Huntingtin-associated protein 1 (HAP1) interacts with the p150Glued subunit of dynactin. Hum Mol Genet 1997; 6:2205–2212.
Gauthier LR, Charrin BC, Borrell-Pages M, et al. Huntingtin controls neurotrophic support and survival of neurons by enhancing BDNF vesicular transport along microtubules. Cell 2004; 118:127–138.
Johansson M, Rocha N, Zwart W, et al. Activation of endosomal dynein motors by stepwise assembly of Rab7-RILP-p150Glued, ORP1L, and the receptor betalll spectrin. J Cell Biol 2007; 176:459–471.
Jordens I, Fernandez-Borja M, Marsman M, et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr Biol 2001; 11:1680–1685.
Cullen PJ . Endosomal sorting and signalling: an emerging role for sorting nexins. Nat Rev Mol Cell Biol 2008; 9:574–582.
Rojas R, Kametaka S, Haft CR, Bonifacino JS . Interchangeable but essential functions of SNX1 and SNX2 in the association of retromer with endosomes and the trafficking of mannose 6-phosphate receptors. Mol Cell Biol 2007; 27:1112–1124.
Xu Y, Hortsman H, Seet L, Wong SH, Hong W . SNX3 regulates endosomal function through its PX-domain-mediated interaction with PtdIns(3)P. Nat Cell Biol 2001; 3:658–666.
Pons V, Luyet PP, Morel E, et al. Hrs and SNX3 functions in sorting and membrane invagination within multivesicular bodies. PLoS Biol 2008; 6:e214.
Traer CJ, Rutherford AC, Palmer KJ, et al. SNX4 coordinates endosomal sorting of TfnR with dynein-mediated transport into the endocytic recycling compartment. Nat Cell Biol 2007; 9:1370–1380.
Yarar D, Waterman-Storer CM, Schmid SL . SNX9 couples actin assembly to phosphoinositide signals and is required for membrane remodeling during endocytosis. Dev Cell 2007; 13:43–56.
Bonifacino JS, Hurley JH . Retromer. Curr Opin Cell Biol 2008; 20:427–436.
Seaman MN . Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 2004; 165:111–122.
Arighi CN, Hartnell LM, Aguilar RC, Haft CR, Bonifacino JS . Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor. J Cell Biol 2004; 165:123–133.
Horazdovsky BF, Davies BA, Seaman MN, et al. A sorting nexin-1 homologue, Vps5p, forms a complex with Vps17p and is required for recycling the vacuolar protein-sorting receptor. Mol Biol Cell 1997; 8:1529–1541.
Seaman MN, McCaffery JM, Emr SD . A membrane coat complex essential for endosome-to-Golgi retrograde transport in yeast. J Cell Biol 1998; 142:665–681.
Griffin CT, Trejo J, Magnuson T . Genetic evidence for a mammalian retromer complex containing sorting nexins 1 and 2. Proc Natl Acad Sci USA 2005; 102:15173–15177.
Wassmer T, Attar N, Bujny MV, et al. A loss-of-function screen reveals SNX5 and SNX6 as potential components of the mammalian retromer. J Cell Sci 2007; 120:45–54.
Carlton J, Bujny M, Peter BJ, et al. Sorting nexin-1 mediates tubular endosome-to-TGN transport through coincidence sensing of high-curvature membranes and 3-phosphoinositides. Curr Biol 2004; 14:1791–1800.
Carlton JG, Bujny MV, Peter BJ, et al. Sorting nexin-2 is associated with tubular elements of the early endosome, but is not essential for retromer-mediated endosome-to-TGN transport. J Cell Sci 2005; 118:4527–4539.
Ishibashi Y, Maita H, Yano M, et al. Pim-1 translocates sorting nexin 6/TRAF4-associated factor 2 from cytoplasm to nucleus. FEBS Lett 2001; 506:33–38.
Hunyady L, Baukal AJ, Gaborik Z, et al. Differential PI 3-kinase dependence of early and late phases of recycling of the internalized AT1 angiotensin receptor. J Cell Biol 2002; 157:1211–1222.
Liu JJ, Ding J, Kowal AS, et al. BPAG1n4 is essential for retrograde axonal transport in sensory neurons. J Cell Biol 2003; 163:223–229.
Gruenberg J . The endocytic pathway: a mosaic of domains. Nat Rev Mol Cell Biol 2001; 2:721–730.
Zerial M, McBride H . Rab proteins as membrane organizers. Nat Rev Mol Cell Biol 2001; 2:107–117.
Rojas R, van Vlijmen T, Mardones GA, et al. Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7. J Cell Biol 2008; 183:513–526.
Zhong Q, Lazar CS, Tronchere H, et al. Endosomal localization and function of sorting nexin 1. Proc Natl Acad Sci USA 2002; 99:6767–6772.
Bravo J, Karathanassis D, Pacold CM, et al. The crystal structure of the PX domain from p40(phox) bound to phosphatidylinositol 3-phosphate. Mol Cell 2001; 8:829–839.
Cozier GE, Carlton J, McGregor AH, et al. The phox homology (PX) domain-dependent, 3-phosphoinositide-mediated association of sorting nexin-1 with an early sorting endosomal compartment is required for its ability to regulate epidermal growth factor receptor degradation. J Biol Chem 2002; 277:48730–48736.
Melkonian KA, Maier KC, Godfrey JE, Rodgers M, Schroer TA . Mechanism of dynamitin-mediated disruption of dynactin. J Biol Chem 2007; 282:19355–19364.
Echeverri CJ, Paschal BM, Vaughan KT, Vallee RB . Molecular characterization of the 50-kD subunit of dynactin reveals function for the complex in chromosome alignment and spindle organization during mitosis. J Cell Biol 1996; 132:617–633.
Wassmer T, Attar N, Harterink M, et al. The retromer coat complex coordinates endosomal sorting and dynein-mediated transport, with carrier recognition by the trans-Golgi network. Dev Cell 2009; 17:110–122.
Ding J, Liu JJ, Kowal AS, et al. Microtubule-associated protein 1B: a neuronal binding partner for gigaxonin. J Cell Biol 2002; 158:427–433.
Acknowledgements
We thank Yingfang Liu (Institute of Biophysics, Chinese Academy of Sciences) for advice on PX domain structure and SNX6 mutations. We are particularly grateful to Yanmin Yang (Stanford University, USA) for insightful discussions and the Flag-MAP1B LC construct. We also thank Juan S Bonifacino (NIH, USA) for the rabbit anti-CI-MPR antibody, Hiroyoshi Ariga (Hokkaido University, Japan) for Flag- and HA-tagged human SNX6 overexpression constructs, and Li Yu (Tsinghua University, China) for the YFP-EEA1 expression construct. We thank Chonglin Yang (Institute of Genetics and Developmental Biology, Chinese Academy of Sciences), Dahua Chen (Institute of Zoology, Chinese Academy of Sciences) and Li Yu for critical reading of the manuscript. This work was supported by grants from the National Natural Science Foundation of China (30770675) and Chinese Academy of Sciences (KSCX1-YW-R-37). J-J Liu is supported by the CAS 100-Talents Program.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information, Table S1
yeast 2-hybrid constructs of SNX6 for mapping of the p150Glued interaction site (PDF 29 kb)
Supplementary information, Table S2
yeast 2-hybrid constructs of p150Glued for mapping of the SNX6 interaction site (PDF 11 kb)
Supplementary information, Figure S1
Immunofluorescence microscopy showing the subcellular localization of SNX6. (PDF 116 kb)
Supplementary information, Movie S1
Supplementary information (AVI 2867 kb)
Supplementary information, Movie S2
Supplementary information (AVI 1371 kb)
Supplementary information, Figure S2
Immunofluorescence microscopy reveals SNX6's colocalization with PI(3)P-enriched endosomal membranes. (PDF 42 kb)
Supplementary information, Figure S3
Wortmannin treatment causes dissociation of both SNX2 and SNX6 from endosomal membranes. (PDF 53 kb)
Supplementary information, Figure S4
Immunofluorescence microscopy showing subcellular distribution of SNX6 mutants. (PDF 79 kb)
Supplementary information, Figure S5
Immunofluorescence microscopy showing subcellular distribution of CI-MPR in SNX1 and SNX6-suppressed cells. (PDF 72 kb)
Supplementary information, Figure S6
Static images showing co-expression of EGFP-tagged p150Glued fragments with mCherry-SNX1 in live cell imaging experiments. (PDF 82 kb)
Supplementary information, Movie S3
Supplementary information (AVI 1468 kb)
Supplementary information, Movie S4
Supplementary information (AVI 2092 kb)
Supplementary information, Movie S5
Supplementary information (AVI 4061 kb)
Rights and permissions
About this article
Cite this article
Hong, Z., Yang, Y., Zhang, C. et al. The retromer component SNX6 interacts with dynactin p150Glued and mediates endosome-to-TGN transport. Cell Res 19, 1334–1349 (2009). https://doi.org/10.1038/cr.2009.130
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cr.2009.130
Keywords
This article is cited by
-
Vesicle trafficking and vesicle fusion: mechanisms, biological functions, and their implications for potential disease therapy
Molecular Biomedicine (2022)
-
Recycling of autophagosomal components from autolysosomes by the recycler complex
Nature Cell Biology (2022)
-
RUFY3 and RUFY4 are ARL8 effectors that promote coupling of endolysosomes to dynein-dynactin
Nature Communications (2022)
-
The intraflagellar transport protein IFT20 controls lysosome biogenesis by regulating the post-Golgi transport of acid hydrolases
Cell Death & Differentiation (2020)
-
Structural and functional insights into sorting nexin 5/6 interaction with bacterial effector IncE
Signal Transduction and Targeted Therapy (2017)