ABSTRACT
Apoptosis or programmed cell death (PCD) is an evolutionarily conserved cellular process that is essential for normal development and homeostasis of multicellular organisms. Defects in the apoptosis signaling result in many diseases including autoimmune diseases and cancer. The apoptosis signaling pathway was first described genetically in the nematode Caenorhabditis elegans which serves as a framework for the more complex apoptotic pathways that exist in mammals. In this review, we will discuss the apoptotic pathways that are emerging in mammals as elucidated by studies of gene-targeted mutant mice.
Similar content being viewed by others
INTRODUCTION
Apoptosis or programmed cell death (PCD) is a genetically programmed cellular event that is conserved throughout evolution. It is a specialized form of cell death that is essential for normal cellular and embryonic development and tissue homeostasis. Defects in apoptosis underlie many human pathological conditions, including cancer, autoimmune diseases and neurodegenerative disorders.
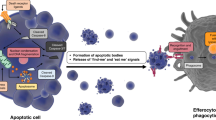
Apoptosis is characterized by specific biochemical and morphological features that culminate in shrinkage of the cell to apoptotic bodies that are engulfed by neighboring macrophages1. The process involves disruptions of mitochondrial, cytoplasmic, and nuclear integrity that result in decreased mitochondrial membrane potential, reversal of phosphatidyl serine to the outer side of the plasma membrane, degradation and “laddering” of DNA, and chromatin condensation. As such, apoptosis is distinct from cell death by necrosis or “accidental cell death”, in which stressed cells swell and burst, releasing cellular contents into the immediate microenvironment. These contents trigger an inflammatory response that can result in tissue damage.
The genetic basis of a signaling pathway leading to PCD was initially identified in the nematode C. elegans2. Since then, our understanding of the mechanisms of PCD has grown exponentially. Through the use of techniques such as the generation of gene-targeted “knockout” mice lacking specific death genes, we have been able to develop mouse models for the elucidation of mammalian PCD pathways in vivo. These mutant animals constitute powerful tools that have the potential to directly influence therapeutic strategies in humans.
Genetic foundation of apoptosis in C. elegans
Four genes in the nematode apoptotic pathway are ced-3, -4, -9, and egl-1 [(ced) cell death abnormal; (egl) egg laying defective] (reviewed in3). ced-3 and ced-4 are the first pro-apoptotic genes identified4. CED-3 is a cysteine protease that cleaves proteins at specific aspartic acid residues5. CED-4 is autoactivated through oligomerization; the activated CED-4 can then activate CED-3 through proteolytic cleavage. The activities of both CED-4 and CED-3 are regulated by CED-9 and EGL-1. CED-9 interferes with the formation of the CED-4: CED-3 complex required for CED-3 activation by binding CED-46. CED-9 can also bind to EGL-17. Once the induction of apoptosis occurs, EGL-1 disrupts the interaction of CED-9 with CED-4 and promotes CED-3 activation8. This appealingly simple pathway, from EGL-1 to CED-9 to CED-4 to CED-3, supplies a framework for the much more complex web of apoptotic pathways found in mammals.
Caspases are the mammalian homologues of ced-3 and at least 14 have been identified to date. Two putative mammalian homologues of ced-4 exist: the apoptotic protease-activating factor-1 (Apaf-1)9 and the Caspase Recruitment Domain4 (CARD4)/Nod-110,11. The mammalian homologues of ced-9 are the anti-apoptotic Bcl-2, Bcl-xL, Bcl-w, and pro-apoptotic Mcl-1 and Bax, Bak and Bok which are members of the Bcl-2 family12. Mammalian homologues of egl-1 are pro-apoptotic BH3-containing members of the Bcl-2 family, including Bik, Bad, Bid, Bim and Bar13, 14.
Caspases: the key players in apoptotic signaling in mammals
Caspases (cysteinyl aspartate-specific proteinases) are mammalian CED-3 homologues that mediate highly specific cleavage events in dying cells. Caspases are expressed as pro-enzymes containing three domains: an NH2 terminal pro-domain, a large subunit (∼20 kD), and a small subunit (∼10 kD). Activation involves proteolytic processing between domains followed by the association of the large and small subunits into an active heterodimer. The active enzymatic site of these proteases is centered around a cysteine residue contained within a conserved QACxG motif. Once activated, the caspases cleave specific aspartic acid residues of proteins involved in the maintenance of nuclear integrity, such as DNA protein kinase (DNA-PK), and poly-ADP-ribose polymerase (PARP), and proteins involved in cell cycle regulation, such as Rb and P21/WAF1. Cleavage by caspases also activates proteins that destroy cell architecture, such as gelsolin which cleaves actin filaments (reviewed in15). Despite many substrates that are identified, the significance and the consequence of many of the cleavage events are not well understood.
There are at least 14 caspases known to date, broadly classified into two subfamilies. The ICE subfamily of proteases, which includes caspase-1, -4, -5 and -11, is primarily involved in processing inflammatory cytokines. The CED-3 subfamily members are known for their roles primarily in apoptosis. Caspase-3 is the prototypical caspase with closest sequence homology and substrate specificity to CED-3. Members of the CED-3 subfamily are further categorized into two groups based on pro-domain characteristics. Caspases in the first group contain long prodomains which facilitate protein-protein interaction and are used to recruit other caspases. For example, caspase-2 and -9 contain the CARD in their prodomains while caspases-8 and -10 have the death effector domain (DED). Through their ability to recruit and activate other caspases, these caspases are thought to play an upstream regulatory role. Caspases in the second group, such as caspase-3, -6, and -7, have short prodomains and are thought to function as downstream “executioner” or effector caspases which cleave substrates resulting in apoptotic cell death (reviewed in16).
Complexity of mammalian apoptosis
In contrast to the simple linear pathway in C. elegans, there are at least two major pathways of apoptosis in mammals. The “extrinsic” pathway is mediated via specific death receptors (DR) expressed on the cell surface. Protein interaction modules such as the DED and others known as death domains (DDs) are used to assemble receptor signaling complex called death-inducing signaling complex (DISC). These complexes recruit and activate the upstream regulatory enzymes, caspase-8 and -10 (reviewed in17 and18), which in turn activate the executioner protease caspase-3. In contrast, the “intrinsic” or mitochondrial pathway of apoptosis is initiated by events in the mitochondria. In response to apoptotic stimuli, in as yet unclarified mechanism, these organelles release cytochrome c into the cytosol which can, in the presence of dATP, associate with Apaf-1 and activate the upstream protease caspase-9. Caspase-9 in turn activates downstream caspases such as caspase-319, 9. Apoptosis-inducing factor (AIF) is also released from the mitochondria upon apoptotic stimulation. AIF can directly translocate to the nucleus and generate large-scale DNA fragmentation in a process which appears to be caspase-independent20, 21. Recently, second mitochondria-derived activator of caspase (Smac) or direct IAP binding protein with low pH (DIABLO) has also been shown to be released from the mitochondria22, 23. It has been shown to be critical for the activation of apoptosis through binding and inhibition of inhibitors of apoptosis (IAPs) (See Fig 1).
The extrinsic pathway of mammalian PCD
Death receptors constitute a TNF receptor superfamily. The prototypical death receptor is Fas (CD95), and Fas-mediated apoptosis will serve as an illustration of the extrinsic pathway for the purposes of this review. Activation of Fas by its ligand FasL results in rapid recruitment of the DD-containing adaptor protein Fas-Associated Death Domain protein (FADD)/Mort-124, 25. Recently it has been shown that these receptors are preformed trimers that undergo conformational changes upon ligand binding that allow activation signaling to occur26. FADD in turn recruits procaspase-8 (also called FLICE (FADD-like IL1-β converting enzyme) or MACH)27, 28. Fas, FADD and procaspase-8 come together at the DISC29, the formation which allows procaspase-8 to oligomerize and activate itself by autocleavage30. Activated caspase-8 then cleaves other procaspases, including procaspase-3, -6, and -731, 32, 33.
DISC also has an inhibitory component, cellular FLICE-inhibitory protein, (cFLIP) [also called CASPER, I-FLICE, CASH, FLAME-1, NRIT, CLARP, or usurpin (reviewed in18). Functional cFLIP contains two DED domains and a caspase-like domain which lacks proteolytic enzymatic activity. Rather, cFLIP molecules bind to the DEDs in FADD and inhibit apoptosis by interfering with the recruitment of procaspase-8 to FADD.
PCD is also held in check by the inhibitor of apoptosis proteins (IAPs). IAPs are the Baculovirus IAP Repeat (BIR)-containing proteins which interfere with the apoptotic pathway by binding to TNF Receptor Associated Factor 2 (TRAF2) as well as caspases (reviewed in34). These inhibitors are therefore not exclusive to the receptor-mediated pathway but can also inhibit the “intrinsic” pathway as they have the capacity to inhibit caspases. IAPs have also been shown to have ubiquitin protein ligase activity which is triggered upon apoptotic stimulation, resulting in autodegradation of the IAP protein and thereby promoting cell death35.
The intrinsic pathway of mammalian PCD
In addition to the signals received through cell surface death receptors, cells also sense, in an as yet unknown way, death signals through the mitochondria. The mitochondrial initiating complex consists of cytochrome c, dATP and Apaf-1, known as the apoptosome36. The apoptosome facilitates PCD through its ability to recruit and activate caspase-9.
Apaf-1 is a 130 kDa protein composed of three functional domains: a short N-terminal CARD, a central CED-4 homology domain, and a long C-terminal “WD-40 repeat” domain9. In the presence of cytochrome c and dATP, Apaf-1 can interact with pro-caspase 9 through their mutual CARDs19. Oligomerization of Apaf-1 in the apoptosome triggers autocatalysis of pro-caspase-9 leading to its activation37. The WD-40 repeats of Apaf-1 serve an important regulatory function because their deletion results in constitutive processing and activation of caspase-9 independent of cytochrome c and dATP37. Other regulatory controls of the intrinsic pathway include the anti-apoptotic Bcl-2 members38 and Bcl-xL39, which can block cytochrome c release, and the pro-apoptotic Bcl-2 members, which can promote the release40, 41.
Lessons from mutant animal models
Much of our present understanding of the apoptotic pathways and their component molecules has come from gain-of-function and loss-of-function studies in animal models. During C. elegans'development, 131 cells normally undergo PCD4. Loss-of-function mutations in ced-3, -4, and egl-1 result in survival of all of the 131 cells, supporting their pro-apoptotic role. However they live a normal life with no obvious deleterious effects. In contrast, ced-9 mutants die early as a consequence of excessive cell death. Gain-of-function mutations in ced-9 block all 131 cell death, supporting its anti-apoptotic role42. In this review, we will discuss the phenotypes of the various mutant mice that have shed light on the roles and importance of individual molecules in the apoptotic pathways.
Analysis of mutants: extrinsic pathway
In mammals, the extrinsic pathway is vital for a normal healthy existence, as is illustrated by the phenotypes of mice mutated in various components of the pathway. lpr and grd mice, which carry spontaneous mutations of the Fas or FasL genes respectively, show dramatic accumulations of lymphocytes resulting in splenomegaly and lymphadenopathy. They exhibit an autoimmune disorder with pathology that is reminiscent of the human autoimmune disease systemic lupus erythematosus (SLE)43. Fas has been shown to be essential for the activation-induced cell death of T cells44 which may account for the lymphoproliferation and the autoimmunity observed in lpr and grd mice.
The phenotypes of mice lacking the extrinsic pathway components FADD, caspase-8 or cFLIP have not only provided in vivo information about the known apoptotic pathway, but have also revealed previously unknown roles for these genes in other cellular processes45, 46, 47. FADD−/− and caspase-8−/− cell lines show resistance to death receptor-mediated PCD whereas cFLIP−/− cell lines are more susceptible, as was expected45, 46. In addition, all three mutants show abnormalities in heart development, an observation that remains unexplained to date. Even though cFLIP appears to have the opposite function to FADD and caspase-8 in the apoptotic pathway, cFLIP−/− embryos exhibit the same heart anomalies as FADD−/− and caspase-8−/− embryos suggesting that in another as yet unidentified pathway, these molecules cooperate with one another.
Analysis of mutants: caspase-3
Caspase-3 is the executioner caspase that serves as one of the key effectors in both the intrinsic and extrinsic pathways. The most dramatic phenotype observed in caspase-3−/− mice is a defect in embryonic brain development that results in the presence of neuronal supernumerary bodies due to a defect in apoptosis and embryonic lethality of some mice starting at day E12.548. Despite the absence of caspase-3, most caspase-3−/− cells can be induced to undergo normal PCD, implying redundancy of function with other executioner caspases. However, caspase-3 is indispensable for the nuclear changes that occur during apoptosis49, although these nuclear hallmarks are not in fact required for PCD50.
The analysis of caspase-3 deficient mice illustrates that components of the death pathways act in a tissue/cell type-specific and stimulus-specific manner. For example, caspase-3−/− embryonic stem (ES) cells die normally following γ-irradiation and heat shock but are resistant to PCD induced by UV-irradiation or hyperosmolarity. caspase-3−/− immature T cells in the thymus are susceptible to Fas-induced cell death but caspase-3−/− mature peripheral T cells are resistant to the same stimulus49.
In addition, caspase-3−/− surviving adult mice injected with agonistic anti-Fas antibody survive longer than wildtype littermates. We can therefore conclude that caspase-3 is indeed required at least partially in Fas-mediated hepatocyte apoptosis. Interestingly, in the absence of caspase-3, cytochrome c release was also impaired, as well as the cleavage of 'upstream'caspases such as caspase-8 and -9. These findings may be explained by the interesting phenomenon that Bcl-2 and Bcl-xL, are cleaved exclusively by caspase-3. We show that caspase-3's role may be expanded to that of a regulator of apoptosis, in addition to its executionary role supported in the previous study51.
Analysis of mutants: the intrinsic pathway
caspase-9 deficient mice show a dramatic brain phenotype similar to that seen in caspase-3−/− embryos52, 53. Activated caspase-3 is absent from caspase-9−/− brains demonstrating that a linear epistatic process operates in this tissue. Caspase-9 is also required for apoptosis of thymocytes, as determined by the resistance of these cells to PCD induced by dexamethasone or γ-irradiation53 However, caspase-9 is not required for extrinsic pathways of PCD such as TNFa- or Fas-mediated cell death53.
Apaf−/− deficient mice exhibit striking cranio-facial abnormalities associated with decreased apoptosis of neuronal cells in the developing brain54, 55. The brain defects observed in apaf-1−/− mice are more profound than those observed in either caspase-9−/− or caspase-3−/− mutants, suggesting that Apaf-1 may be involved in apoptotic pathways other than those involving caspase-3 or caspase-9. Mutation of Apaf-1, but not caspase-3 or caspase-9, results in a delay in the removal of the embryonic interdigital webs, and in abnormal eye development. Furthermore, similar to caspase-9 deficiency, apaf-1−/− cells are resistant to a wide variety of apoptotic stimuli, including γ-irradiation, dexamethasone and chemotherapeutic agents but not from death receptor-mediated killing.
Embryos devoid of cytochrome c are not viable beyond midgestation. Nevertheless, development up to this stage appears to progress in a relatively normal but delayed manner56. cytochrome c-deficient cultured cells from living embryos display respiratory insufficiency and enhanced anaerobic glycolysis, manifested by slowed growth and rapid acidification of the culture medium. cytochrome c−/− embryonic cells are more resistant to apoptosis induced by UV-irradiation, serum starvation or staurosporin. In contrast, apoptosis in response to TNFα is enhanced in the absence of cytochrome c. This interesting observation suggests a previously unrecognized interaction between the instrinsic and extrinsic pathways, in which a defect in one upregulates the other.
Characterization of caspase-2−/− mice found these mice to have an excess number of germ cells in their ovaries that are resistant to chemotherapeutic agents. On the other hand, caspase-2−/− sympathetic neurons are more sensitive to apoptosis57. Caspase-12 has been shown to be required specifically for endoplasmic reticulum (ER) stress mediated apoptosis58.
Crosstalk between the two pathways
The seemingly distinct pathways described so far do not always occur independently in vivo. For example, although in some cell types, such as T lymphocytes, ligation of Fas via FADD and caspase-8 can induce effector caspase activation and culminate in cell death without affecting the mitochondria as shown in Apaf-1 and caspase-9 komic. However, the mitochondrial pathway is involved upon death receptor triggering in some situations. In the scenario where FADD and caspase-8 are sufficient for Fas-mediated apoptosis28,27, cytochrome c is not released and effector caspases are directly activated early on59. In this mitochondria-independent pathway, cell death is not inhibitable by Bcl-2 but rather by direct caspase inhibitors such as CrmA59. However, in other cell types, such as hepatocytes, apoptotic induction by Fas ligation results in cytochrome c release and the process is inhibitable by Bcl-2, implying the involvement of a mitochondrial pathway. One protein that may account for this discrepancy is a BH3 containing Bcl-2 family member called Bid60,61.
Bid is cleaved by caspase-8 and the cleaved carboxy terminal peptide (tBID) is targeted to the mitochondria where it triggers cytochrome c release62. Bid−/− hepatocytes exhibit a defect in cytochrome c release and resistance to apoptosis, thereby illustrating that the mitochondrial pathway is required for amplifying death signals63.
Other studies show that tBid can somehow cause a change in the conformational state of Bax. This results in oligomerization of Bax and facilitates its insertion into the outer mitochondrial membrane, triggering mitochondrial dysfunction and subsequent cytochrome c release64. Another group has shown that tBid is also able to induce a conformational change and oligomerization of Bak65. It is postulated that different cell types use different pro-apoptotic Bcl-2 proteins.
Novel roles for caspases
Some recent studies have suggested that caspases may be involved in cellular processes other than apoptosis. Caspase-3 and -8 have been shown to be important for T cell proliferation66, 67, and caspase-3 may play a role in cell cycle regulation in splenic B cells. In vitro, caspase-3 can cleave numerous proteins involved in variety of pathways, including molecules important for cell cycle and survival signaling68. We have shown that caspase-3−/− splenic B cells have an intrinsic capacity to hyperproliferate in the absence of a cell death defect. Indeed, caspase-3−/− B cells have a greater clonogenic capacity and show a higher proportion of cells in S phase than wild type B cells. These data suggest that caspase-3 may be required for cell cycle arrest rather than for apoptosis in certain cell types (Data not shown/Manuscript in preparation).
CONCLUSION
At present, most of our knowledge of PCD signaling pathways is at the cellular level. PCD and its interaction with other processes is much more complex at the organism level. It is imperative that we understand these mechanisms in vivo because so many human diseases result as a consequence of defects in PCD regulation. Once the cellular mechanisms of cell death are understood in vivo, we can devise more effective therapeutic strategies for disease prevention and progression.
References
Wyllie AH, Kerr JF, Currie AR . Cell death: the significance of apoptosis. Int Rev Cytol 1980; 68:251–306.
Ellis HM, Horvitz HR . Genetic control of programmed cell death in the nematode C. elegans. Cell 1986; 44:817–29.
Vaux DL, Korsmeyer SJ . Cell death in development. Cell 1999; 96:245–54.
Yuan JY, Horvitz HR . The Caenorhabditis elegans genes ced-3 and ced-4 act cell autonomously to cause programmed cell death. Dev Biol 1990; 138:33–41.
Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR . The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 b-converting enzume. Cell 1993; 75:641–52.
Cain K, Bratton SB, Langlais C et al. Apaf-1 oligomerized into biologically active approximately 700-kDa and inactive approximately 1. 4-MDa apoptosome complexes. J Biol Chem 2000; 275:6067–70.
Conradt B, Horvitz HR . The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 1998; 93:519–29.
del Peso L, Gonzalez VM, Nunez G . Caenorhabditis elegans EGL-1 disrupts the interaction of CED-9 with CED-4 and promotes CED-3 activation. J Biol Chem 1998; 273:33495–500.
Zou H, Henzel WJ, Liu X, Lutschg A, Wang X . Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3 [see comments]. Cell 1997; 90:405–13.
Bertin J, Nir WJ, Fischer CM et al. Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kB. J Biol Chem 1999; 274:12955–8.
Inohara N, Koseki T, del Peso L et al. Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kB. J Biol Chem 1999; 274:14560–7.
Adams JM, Cory S . The Bcl-2 protein family: arbiters of cell survival. Science 1998; 281:1322–6.
Reed JC . Bcl-2 family proeins. Oncogene 1998; 17:3225–36.
Zhang H, Xu Q, Krajewski S et al. An apoptosis regulator at the intersection of caspases and Bcl-2 family proteins. Proc Natl Acad Sci USA 2000; 97:2597–602.
Thornberry Na, Lazebnik Y . Caspases: enemies within. Science 1998; 281:1312–6.
Nicholson DW, Thornberry NA . Caspases: killer proteases. Trends Biochem Sci 1997; 22:299–306.
Scaffidi C, Kirchhoff S, Krammer PH, Peter ME . Apoptosis signaling in lymphocytes. Curr Opin Immunol 1999; 11:277–85.
Ashkenazi A, Dixit VM . Apoptosis control by death and decoy receptors. Curr Opin Cell Biol 1999; 11:255–60.
Liu X, Kim CN, Yang J, Jemmerson R, Wang X . Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 1996; 86:147–57.
Susin SA, Lorenzo HK, Zamzami N et al. Molecular characterization of mitochondrial apoptosis-inducing factor [see comments]. Nature 1999; 397:441–6.
Daugas E, Susin SA, Zamzami N et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. Faseb J 2000; 14:729–39.
Verhagen AM, Ekert PG, Pakusch M et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 2000; 102:43–53.
Du C, Fang M, Li Y, Li L, Wang X . Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 2000; 102:33–42.
Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D . A novel protein that interacts with the death domain of Fas/APO1 contains a sequence motif related to the death domain. J Biol Chem 1995; 270:7795–8.
Chinnaiyan AM, O'Rourke K, Tewari M, Dixit VM . FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell 1995; 81:505–12.
Siegel RM, Frederiksen JK, Zacharias DA et al. Fas preassociation required for apoptosis signaling and dominant inhibition by pathogenic mutations [see comments]. Science 2000; 288:2354–7.
Boldin M P, Goncharov TM, Goltsev YV, Wallach D . Involvement of MACH, a novel MORT1/ FADD-interacting protease, in Fas/APO1- and TNF receptor-induced cell death. Cell 1996; 85:803–15.
Muzio M, Chinnaiyan AM, Kischkel FC et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell 1996; 85:817–27.
Kischkel FC, Hellbardt S, Behrmann I et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. An induced proximity model for caspase-8 activation. Embo J 1995; 14:5579–88.
Muzio M, Stockwell BR, Stennicke HR, Salvesen GS, Dixit VM . An induced proximity model for caspase-8 activation. J Biol Chem 1998; 273:2926–30.
Enari M, Talanian RV, Wong WW, Nagata S . Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature 1996; 380:723–6.
Takahashi A, Hirata H, Yonehara S et al. Affnity labeling displays the stepwise activation of ICE-related proteases by Fas, staurosporine, and CrmA-sensitive caspase-8. Oncogene 1997; 14:2741–52.
Fernandes-Alnemri T, Litwack G, Alnemri ES . Mch2, a new member of the apoptotic Ced-3/Ice cysteine protease gene family. Cancer Res 1995; 55:2737–42.
Miller LK . An exegesis of IAPs: salvation and surprises from BIR motifs. Trends Cell Biol 1999; 9:323–8.
Yang Y, Fang S, Jensen JP, Weissman AM, Ashwell JD . Ubiquitin protein ligase activity of IAPs and their degradation in in protesomes in response to apoptotic stimuli. Science 2000; 288:874–7.
Budihardjo I, Oliver H, Lutter M, Luo X, Wang X . Biochemical pathways of caspase activation during apoptosis. Annu Rev Cell Dev Biol 1999; 15:269–90.
Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES . Autoactivation of procaspase-9 by APaf-1-mediated oligomerization. Mol Cell 1998; 1:949–57.
Yang X, Chang HY, Baltimore D . Essential role of CED-4 oligomerization in CED-3 activation and apoptosis [see comments]. Science 1998; 281:1355–7.
Li F, Srinivasan A, Wang Y, Armstrong RC, Tomaselli KJ, Fritz LC . Cell-specific induction of apoptosis by microinjection of cytochrome c Bcl-xL has activity independent c of cytochrome c release. J Biol Chem 1997; 272:30299–305.
Rosse T, Olivier R, Monney L et al. Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c [see comments]. Nature 1998; 391:496–9.
Jurgensmeier JM, Xie Z, Deveraux Q, Ellerby L, Bredesen D, Reed JC . Bax directly induces release of cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA 1998; 95:4997–5002.
Meier P, Finch A, Evan G . Apoptosis in development [In Process Citation]. Nature 2000; 407:796–801.
Nagata S . Apoptosis by death factor. Cell 1997; 88:355–65.
Akbar AN, Salmon M . Cellular environments and apoptosis: tissue microenvironments control activated T-cell death. Immunol Today 1997; 18:72–6.
Yeh WC, Pompa JL, McCurrach ME et al. FADD: essential for embryo development and signaling from some, but not all, inducers of apoptosis. Science 1998; { 279:1954–8.
Varfolomeev EE, Schuchmann M, Luria V et al. Targeted disruption of the mouse Caspase 8 gene ablates cell death induction by the TNF receptors, Fas/Apo1, and DR3 and is lethal prenatally. Immunity 1998; 9:267–76.
Yeh WC, Itie A, Elia AJ et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development [In Process Citation]. Immunity 2000; 12:633–42.
Kuida K, Zheng TS, Na S et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature 1996; 384:368–72.
Woo M, Hakem R, Soengas MS et al. Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev 1998; 12:806–19.
Zhang J, Liu X, Scherer DC, van Kaer L, Wang X, Xu M . Resistamce to DNA fragmentation and chromatin condensation in mice lacking the DNA fragmentation factor 45. Proc Natl Acad Sci USA 1998; 95:12480–5.
Woo M, Hakem A, Elia AJ et al. In vivo evidence that caspase-3 is required for Fas-mediated apoptosis of hepatocytes. J Immunol 1999; 163:4909–16.
Kuida K, Haydar TF, Kuan CY et al. Re]uced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell 1998; 94:325–37.
Hakem R, Hakem A, Duncan GS et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell 1998; 94:339–52.
Yoshida H, Kong YY, Yoshida R et al. Apaf1 is rwquired for mitochondrial pathways of apoptosis and brain development. Cell 1998; 94:739–50.
Cecconi F, Alvarez-Bolado G, Meyer BI, Roth KA, Gruss P . Apaf1 (CED-4 homolog) regulates programmed cell deathin mammalian development. Cell 1998; 94:727–37.
Li K, Li Y, Shelton JM et al. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell 2000; 101:389–99.
Bergeron L, Perez GI, Macdonald G et al. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-b. Genes Dev 1998; 12:1304–14.
Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J . Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxicity by amyloid-b. Nature 2000; 403:676.5–103.
Scaffidi C, Fulda S, Srinivasan A et al. Two CD95 (APO-1/Fas) signaling pathways. Embo J 1998; 17:1675–87.
Luo X, Budihardjo I, Zou H, Slaughter C, Wang X . Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 1998; 94:481–90.
Li H, Zhu H, Xu CJ, Yuan J . Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998; 94:491–501.
Gross A, Yin XM, Wang K et al. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-xL prevents this release but not tumor necrosis factor-R1/Fas death. J Biol Chem 1999; 274
Yin XM, Wang K, Gross A et al. Bid-deficient mice are resistant to Fas-inducde hepatocellular apoptosis. Nature 1999; 400:886–91.
Eskes R, Desagher S, Antonsson B, Martinou JC . Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol 2000; 20:929–35.
Wei MC, Lindsten T, Mootha VK et al. tBid, a membrane-targeted death ligand, oligomerized BAK to release cytochrome c. Genes Dev 2000; 14:2060–71.
Alam A, Cohen LY, Aouad S, Sekaly RP . Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells. J Exp Med 1999; 190:1879–90.
Kennedy N J, Kataoka T, Tschopp J, Budd RC . Caspase activation is required for T cell proliferation. J Exp Med 1999; 190:1891–6.
Widmann C, Gibson S, Johnson GL . Caspase-dependent cleavage of signaling proteins during apoptosis. A turn-off mechanism for anti-apoptotic signals. J Biol Chem 1998; 273:7141–7.
Acknowledgements
We are grateful to Mary Saunders for scientific editing. MW is supported by a fellowship from the Medical Research Council of Canada (MRC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
WOO, M., HAKEM, R. & MAK, T. Executionary pathway for apoptosis: lessons from mutant mice. Cell Res 10, 267–278 (2000). https://doi.org/10.1038/sj.cr.7290054
Issue Date:
DOI: https://doi.org/10.1038/sj.cr.7290054
Keywords
This article is cited by
-
TLR9 agonist enhances radiofrequency ablation-induced CTL responses, leading to the potent inhibition of primary tumor growth and lung metastasis
Cellular & Molecular Immunology (2019)
-
Molecular Pathway of Psoralidin-Induced Apoptosis in HepG2 Cell Line
Chinese Journal of Integrative Medicine (2019)
-
Influence of selected anti-cancer drugs on the induction of DNA double-strand breaks and changes in gene expression in human hepatoma HepG2 cells
Environmental Science and Pollution Research (2016)
-
Immunogenicity of necrotic cell death
Cellular and Molecular Life Sciences (2015)
-
Nuclear translocation and accumulation of glyceraldehyde-3-phosphate dehydrogenase involved in diclazuril-induced apoptosis in Eimeria tenella (E. tenella)
Veterinary Research (2013)