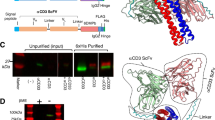
Abstract
CD28 is one of the costimulatory molecules crucial for T-cell activation and thus has become an attractive target for therapeutic immunomodulation. Conventional strategies for blocking CD28 activity using monoclonal antibodies, Fab fragments, antagonistic peptide and fusion proteins, have apparent disadvantages such as inherent immunogenicity, unwanted Fc signaling, poor tissue penetration and bioinstability. Recent research has been directed toward the creation of non-natural, sequence-specific biomimetic oligomers with bioinspired structures that capture the amino-acid interface of the targeted proteins. One such family of molecules is the poly-N-substituted glycines or peptoids, which have close structural similarity to peptides but are essentially invulnerable to protease degradation. To screen for peptoids that specifically target CD28, we first designed and chemically synthesized 19 candidate peptoids based on molecular modeling and docking. Using the phage-displaying system that expresses the extracellular domain of the CD28 homodimer and contains the core B7-binding motif, a peptoid (No. 9) with a molecular formula of C21H29N3O7, was identified to display the highest binding activity to CD28. This peptoid not only inhibited the lymphocyte proliferation in vitro, but suppressed immunoresponses against alloantigens in vivo, and attenuated the graft-versus-host disease in a mouse bone-marrow transplantation model. These results suggested that peptoids targeting CD28 are effective agents for blocking the CD28-mediated costimulation and suitable for development of novel therapeutic approaches for diseases involving this pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Schwartz RH . A cell culture model for T lymphocyte clonal anergy. Science 1990; 258: 1349.
June CH, Ledbetter JA, Linsley PS, Thompson CB . Role of the CD28 receptor in T-cell activation. Immunol Today 1990; 11: 211.
Shahinian A, Pfeffer K, Lee KP, Kündig TM, Kishihara K, Wakeham A et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science 1993; 261: 609.
Thompson CB, Lindsten T, Ledbetter JA, Kunkel SL, Young HA, Emerson SG et al. CD28 activation pathway regulates the production of multiple T-cell-derived lymphokines/cytokines. Proc Natl Acad Sci USA 1989; 86: 1333.
June CH, Bluestone JA, Nadler LM, Thompson CB . The B7 and CD28 receptor families. Immunol Today 1994; 15: 321.
Lenschow DJ, Walunas TL, Bluestone JA . CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996; 14: 233.
Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP . CD28-mediated signaling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature 1992; 356: 607.
Gimmi CD, Freeman GJ, Gribben JG, Gray G, Nadler LM . Human T-cell clonal anergy is induced by antigen presentation in the absence of B7 costimulation. Proc Natl Acad Sci USA 1993; 90: 6586.
Linsley PS, Brady W, Urnes M, Grosmaire LS, Damle NK, Ledbetter JA . CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med 1991; 174: 561–569.
Sharpe AH, Freeman GJ . The B7-CD28 superfamily. Nat Rev Immunol 2002; 2: 116–126.
Miller SD, Vanderlugt CL, Lenschow DJ . Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapse of murine EAE. Immunity 1995; 3: 739–745.
Newell KA, He G, Guo Z, Kim O, Szot GL, Rulifson I et al. Cutting edge: blockade of the CD28/B7 costimulatory pathway inhibits intestinal allograft rejection mediated by CD4+ but not CD8+ T cells. J Immunol 1999; 163: 2358–2362.
Racke MK, Scott DE, Quigley L, Gray GS, Abe R, June CH et al. Distinct roles for B7-1 (CD-80) and B7-2 (CD-86) in the initiation of experimental allergic encephalomyelitis. J Clin Invest 1995; 96: 2195.
Perrin PJ, Scott D, Davis TA, Gray GS, Doggett MJ, Abe R et al. Opposing effects of CTLA4-Ig and anti-CD80 (B7-1) plus anti-CD86 (B7-2) on experimental allergic encephal-omyelitis. J Neuroimmunol 1996; 65: 31.
Perrin PJ, June CH, Maldonado JH, Ratts RB, Racke MK . Blockade of CD28 during in vitro activation of encephalitogenic T cells or after disease onset ameliorates experimental autoimmune encephalomyelitis. J Neuroimmunol 1999; 163: 1704–1710.
Marx V . Watching peptide drugs grow up. Chem Eng News 2005; 83: 17–24.
Caudic P, Stawikowski M . Peptidomimetics: Fmoc solid-phase pseudopeptide synthesis. Methods Mol Biol 2008; 494: 223–246.
Kirshenbaum K, Zuckermann RN, Dill KA . Designing polymers that mimic biomolecules. Curr Opin Struct Biol 1999; 9: 530–535.
Gellman SH . Foldamers: a manifesto. Acc Chem Res 1998; 31: 173–180.
Barron AE, Zuckermann RN . Bioinspired polymeric materials: in-between plastics and proteins. Curr Opin Chem Biol 1999; 3: 681–687.
Figliozzi GM, Goldsmith R, Ng SC, Banville SC, Zuckermann RN . Synthesis of N-substituted glycine peptoid libraries. Methods Enzymol 1996; 267: 437–447.
Kirshenbaum K, Barron AE, Goldsmith RA, Armand P, Bradley EK, Truong KT et al. Sequence-specific polypeptoids: a diverse family of heteropolymers with stable secondary structure. Proc Natl Acad Sci USA 1998; 95: 4303–4308.
Sayegh MH, Turka LA . The role of T-cell costimulatory activation pathways in transplant rejection. N Engl J Med 1998; 338: 1813–821.
Larsen CP, Elwood ET, Alexander DZ, Ritchie SC, Hendrix R, Tucker-Burden C et al. Long term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature 1996; 381: 434–438.
Lechler R, Sykes M, Thomson AW, Turka LA . Organ transplantation-how much of the promise has been realized. Nat Med 2005; 11: 605–613.
Khoury SJ, Sayegh MH . The roles of the new negative T-cell costimulatory pathways in regulating autoimmunity. Immunity 2004; 20: 529–538.
Li XC, Rothstein DM, Sayegh MH . Costimulatory pathways in transplantation: challenges and new developments. Immunol Rev 2009; 229: 271–293.
Barbas CF III, Wagner J . Synthetic human antibodies: selecting and evolving functional proteins. Methods 1995; 8: 94–103.
Tayapiwatana C, Kasinrerk W . Construction and characterization of phage-displayed leukocyte surface molecule CD99. Appl Microbiol Biotechnol 2002; 60: 336–341.
Tayapiwatana C, Arooncharus P, Kasinrerk W . Displaying and epitope mapping of CD147 on VCSM13 phages: influence of Escherichia coli strains. J Immunol Methods 2003; 281: 177–185.
Manosroi J, Tayapiwatana C, Gotz F, Werner RG, Manosroi A . Secretion of active recombinant human tissue plasminogen activator derivatives in Escherichia coli. Appl Environ Microbiol 2001; 67: 2657–2664.
Acknowledgements
This work was supported by grants from the Natural Science Foundation of China (No. 30628015 and No. 30872309) and National 973 Program (No. 2006CB503803).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Li, N., Zhu, F., Gao, F. et al. Blockade of CD28 by a synthetical peptoid inhibits T-cell proliferation and attenuates graft-versus-host disease. Cell Mol Immunol 7, 133–142 (2010). https://doi.org/10.1038/cmi.2009.120
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/cmi.2009.120
Keywords
This article is cited by
-
Overcoming immunological barriers in regenerative medicine
Nature Biotechnology (2014)