Abstract
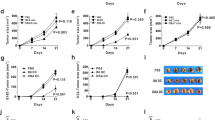
As they should enhance tumor-specific antigen presentation by dendritic cells, tumor cell lines genetically modified to express CD154 molecules have been used in an attempt to induce protective antitumor immunity. Two murine models were used: the major histocompatibility complex (MHC) class I negative melanoma B16F10 and the MHC class I positive mammary adenocarcinoma TS/A. CD154 or mock-transfected B16F10 or TS/A cells were injected subcutaneously into H-2–compatible B6D2 mice. CD154 expression by tumor cells induced a complete rejection (in the TS/A model) or a striking reduction (in the B16F10 model) of modified tumors growth, but also a significant protection against the growth of mock tumor cells injected simultaneously, either mixed with the CD154-expressing tumor cells, or in the other flank of mice. Thirty days after CD154-expressing tumor rejection, splenic lymphocytes from surviving tumor-free mice were able to inhibit tumor proliferation in vitro and significant amounts of IFN-γ were detected in the sera of these mice. Growth kinetics of mock and CD154-expressing tumors in immunocompetent versus nude mice suggest that T lymphocytes and natural killer cells responses are implicated in this antitumor immunity. The injection of CD154-expressing tumor cell induced an antitumor protective response, both locally and distant from the injection site. The effect was most pronounced in MHC class I expressing TS/A tumor model.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Van den Eynde BJ, van der Bruggen P . T cell defined tumor antigens Curr Opin Immunol 1997 9: 684
Couderc B, Zitvogel L, Douin-Echinard V et al. Enhancement of antitumor immunity by expression of CD70 (CD27 ligand) or CD154 (CD40 ligand) costimulatory molecules in tumor cells Cancer Gene Ther 1998 5: 163
Williams IR, Ort RJ, Daley D et al. Constitutive expression of B7-1 (CD80) on mouse keratinocytes does not prevent development of chemically induced skin papillomas and carcinomas J Immunol 1996 156: 3382
Douin-Echinard V, Robbins PD, Lotze MT, Favre G, Couderc B . Enhancement of anti-tumor immunity by injection of fibroblasts genetically engineered to produce IL-12 and to express CD70 Adv Exp Med Biol 1998 451: 353
Nieland JD, Graus YF, Dortmans YE, Kremers BL, Kruisbeek AM . CD40 and CD70 co-stimulate a potent in vivo antitumor T cell response J Immunother 1998 21: 225
Toes REM, Schoenberger SP, van der Voort EIH, Offringa R, Melief CJM . CD40–CD40 ligand interactions and their role in cytotoxic T lymphocyte priming and anti-tumor immunity Semin Immunol 1998 10: 443
Moodycliffe AM, Shreedhar V, Ullrich SE et al. CD40–CD40 ligand interactions in vivo regulate migration of antigen-bearing dendritic cells from the skin to draining lymph nodes J Exp Med 2000 191: 2011
Schuurhuis DH, Laban S, Toes RE et al. Immature dendritic cells acquire CD8(+) cytotoxic T lymphocyte priming capacity upon activation by T helper cell–independent or –dependent stimuli J Exp Med 2000 192: 145
Banchereau J . Dendritic cells: therapeutic potentials Transfus Sci 1997 18: 313
Schoenberger SP, Toes RE, van der Voort EI, Offringa R, Melief CJ . T-cell help for cytotoxic T lymphocytes is mediated by CD40–CD40L interactions [see comments] Nature 1998 393: 480
Eggert AA, Schreurs MW, Boerman OC et al. Biodistribution and vaccine efficiency of murine dendritic cells are dependent on the route of administration Cancer Res 1999 59: 3340
Zitvogel L, Mayordomo JI, Tjandrawan T et al. Therapy of murine tumors with tumor peptide–pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1–associated cytokines [see comments] J Exp Med 1996 183: 87
Morse MA, Deng Y, Coleman D et al. A Phase I study of active immunotherapy with carcinoembryonic antigen peptide (CAP-1)–pulsed, autologous human cultured dendritic cells in patients with metastatic malignancies expressing carcinoembryonic antigen Clin Cancer Res 1999 5: 1331
Nestle FO, Alijagic S, Gilliet M et al. Vaccination of melanoma patients with peptide- or tumor lysate–pulsed dendritic cells [see comments] Nat Med 1998 4: 328
Grohmann U, Belladonna ML, Bianchi R et al. Immunogenicity of tumor peptides: importance of peptide length and stability of peptide/MHC class II complex Cancer Immunol Immunother 1999 48: 195
Kugler A, Stuhler G, Walden P et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell–dendritic cell hybrids [see comments] Nat Med 2000 6: 332
Douin-Echinard V, Bornes S, Rochaix P et al. The expression of CD70 and CD80 by gene-modified tumor cells induces an antitumor response depending on the MHC status Cancer Gene Ther 2000 7: 1543
Terheyden P, Straten P, Brocker EB, Kampgen E, Becker JC . CD40-ligated dendritic cells effectively expand melanoma-specific CD8+ CTLs and CD4 + IFN-gamma–producing T cells from tumor-infiltrating lymphocytes J Immunol 2000 164: 663
Giacomini P, Tecce R, Gambari R, Sacchi A, Fisher PB, Natali PG . Recombinant human IFN-gamma but not IFN-alpha or IFN-beta, enhances MHC– and non-MHC–encoded glycoproteins by a protein synthesis–dependent mechanism J Immunol 1988 140: 3073
Klein C, Bueler H, Mulligan RC . Comparative analysis of genetically modified dendritic cells and tumor cells as therapeutic cancer vaccines J Exp Med 2000 191: 1699
Kikuchi T, Moore MA, Crystal RG . Dendritic cells modified to express CD40 ligand elicit therapeutic immunity against preexisting murine tumors Blood 2000 96: 91
Guilloux Y, Bai XF, Liu X, Zheng P, Liu Y . Optimal induction of effector but not memory antitumor cytotoxic T lymphocytes involves direct antigen presentation by the tumor cells Cancer Res 2001 61: 1107
Acknowledgements
This work was supported by grants from the Claudius Regaud Institut and the Association for Research against Cancer. We thank Marie Penary and Danièle Berg for excellent biotechnical assistance, and Christiane Pages and Lourdes Gasquet from the animal facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grangeon, C., Cormary, C., Douin-Echinard, V. et al. In vivo induction of antitumor immunity and protection against tumor growth by injection of CD154-expressing tumor cells. Cancer Gene Ther 9, 282–288 (2002). https://doi.org/10.1038/sj.cgt.7700439
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700439
Keywords
This article is cited by
-
Coexpression of CD40L and CD70 by semiallogenic tumor cells induces anti-tumor immunity
Cancer Gene Therapy (2005)
-
Induction of T-cell antitumor immunity and protection against tumor growth by secretion of soluble human CD70 molecules
Cancer Gene Therapy (2004)
-
Concurrent delivery of tumor antigens and activation signals to dendritic cells by irradiated CD40 ligand-transfected tumor cells resulted in efficient activation of specific CD8+ T cells
Cancer Gene Therapy (2004)
-
The role of CD40-CD154 interaction in cell immunoregulation
Journal of Biomedical Science (2004)