Abstract
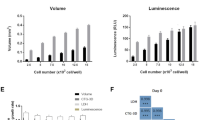
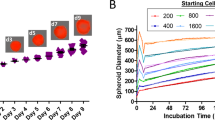
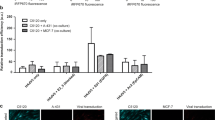
Oncolytic viruses represent a novel cancer treatment strategy. Despite their promising preclinical data, however, corresponding clinical trials have disappointed. To aid preclinical analyses, we hypothesized that three-dimensional tumor cell clusters or spheroids might provide an assay system superior to conventional monolayer cell cultures. Spheroids show viral infection, replication and oncolytic patterns distinct from conventional monolayer assays. Therefore, viral tumor penetration and oncolysis measurements may be improved with such three-dimensional models. Also, preclinical analyses of oncolytic viruses frequently measure mitochondrial activity, but more accurate measures of oncolysis might involve quantitation of intracellular protein release. Therefore, we measured luciferase released from luciferase-expressing spheroids and found unique patterns that maintained consistency with various viruses and doses. The relative variations between viruses and doses may represent temporal differences in oncolysis dynamics. Analysis of five recombinant replicative adenoviruses with promise for clinical application showed that Ad5/3-Δ24 produced the most luciferase release 1 week after infection and achieved the earliest and highest peak luciferase release level. Ad5/3-Δ24 also effected the earliest subtotal spheroid cell death. These findings closely parallel monolayer oncolysis assays with these agents. Therefore, the luciferase-expressing tumor spheroid assay represents a promising three-dimensional model for preclinical analysis of replicative oncolytic agents.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Kirn D, Martuza RL, Zwiebel J . Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med 2001; 7: 781–787.
Kanerva A, Hemminki A . Modified adenoviruses for cancer gene therapy. Int J Cancer 2004; 110: 475–480.
Theys J, Barbe S, Landuyt W, Nuyts S, Van Mellaert L, Wouters B et al. Tumor-specific gene delivery using genetically engineered bacteria. Curr Gene Ther 2003; 3: 207–221.
Puumalainen AM, Vapalahti M, Agrawal RS, Kossila M, Laukkanen J, Lehtolainen P et al. Beta-galactosidase gene transfer to human malignant glioma in vivo using replication-deficient retroviruses and adenoviruses. Hum Gene Ther 1998; 9: 1769–1774.
Lamfers ML, Hemminki A . Multicellular tumor spheroids in gene therapy and oncolytic virus therapy. Curr Opin Mol Ther 2004; 6: 403–411.
Kanerva A, Hemminki A . Adenoviruses for treatment of cancer. Ann Med 2005; 37: 33–43.
Saukkonen K, Hemminki A . Tissue-specific promoters for cancer gene therapy. Expert Opin Biol Ther 2004; 4: 683–696.
Yamamoto M, Davydova J, Wang M, Siegal GP, Krasnykh V, Vickers SM et al. Infectivity enhanced, cyclooxygenase-2 promoter-based conditionally replicative adenovirus for pancreatic cancer. Gastroenterology 2003; 125: 1203–1218.
Kanerva A, Bauerschmitz GJ, Yamamoto M, Lam JT, Alvarez RD, Siegal GP et al. A cyclooxygenase-2 promoter-based conditionally replicating adenovirus with enhanced infectivity for treatment of ovarian adenocarcinoma. Gene Therapy 2004; 11: 552–559.
Suzuki K, Fueyo J, Krasnykh V, Reynolds PN, Curiel DT, Alemany R . A conditionally replicative adenovirus with enhanced infectivity shows improved oncolytic potency. Clin Cancer Res 2001; 7: 120–126.
Kanerva A, Zinn KR, Chaudhuri TR, Lam JT, Suzuki K, Uil TG et al. Enhanced therapeutic efficacy for ovarian cancer with a serotype 3 receptor-targeted oncolytic adenovirus. Mol Ther 2003; 8: 449–458.
Bauerschmitz GJ, Lam JT, Kanerva A, Suzuki K, Nettelbeck DM, Dmitriev I et al. Treatment of ovarian cancer with a tropism modified oncolytic adenovirus. Cancer Res 2002; 62: 1266–1270.
Kanerva A, Zinn KR, Peng KW, Ranki T, Kangasniemi L, Chaudhuri TR et al. Noninvasive dual modality in vivo monitoring of the persistence and potency of a tumor targeted conditionally replicating adenovirus. Gene Therapy 2005; 12: 87–94.
Kirn D . Clinical research results with dl1520 (Onyx-015), a replication-selective adenovirus for the treatment of cancer: what have we learned? Gene Therapy 2001; 8: 89–98.
Lam JT, Bauerschmitz GJ, Kanerva A, Barker SD, Straughn JM, Wang M et al. Replication of an integrin targeted conditionally replicating adenovirus on primary ovarian cancer spheroids. Cancer Gene Therapy 2003; 10: 377–387.
Lam JT, Kanerva A, Bauerschmitz GJ, Takayama K, Suzuki K, Yamamoto M et al. Inter-patient variation in efficacy of five oncolytic adenovirus candidates for ovarian cancer therapy. J Gene Med 2004; 6: 1333–1342.
Schafer H, Schafer A, Kiderlen AF, Masihi KN, Burger R . A highly sensitive cytotoxicity assay based on the release of reporter enzymes, from stably transfected cell lines. J Immunol Methods 1997; 204: 89–98.
Hemminki A, Belousova N, Zinn KR, Liu B, Wang M, Chaudhuri TR et al. An adenovirus with enhanced infectivity mediates molecular chemotherapy of ovarian cancer cells and allows imaging of gene expression. Mol Ther 2001; 4: 223–231.
Hemminki A, Zinn KR, Liu B, Chaudhuri TR, Desmond RA, Rogers BE et al. In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst. 2002; 94: 741–749.
Krasnykh V, Belousova N, Korokhov N, Mikheeva G, Curiel DT . Genetic targeting of an adenovirus vector via replacement of the fiber protein with the phage T4 fibritin. J Virol 2001; 75: 4176–4183.
Mittereder N, March KL, Trapnell BC . Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol 1996; 70: 7498–7509.
Kunz-Schughart LA, Kreutz M, Knuechel R . Multicellular spheroids: a three-dimensional in vitro culture system to study tumour biology. Int J Exp Pathol 1998; 79: 1–23.
Douglas JT, Kim M, Sumerel LA, Carey DE, Curiel DT . Efficient oncolysis by a replicating adenovirus (ad) in vivo is critically dependent on tumor expression of primary ad receptors. Cancer Res 2001; 61: 813–817.
Hemminki A, Dmitriev I, Liu B, Desmond RA, Alemany R, Curiel DT . Targeting oncolytic adenoviral agents to the epidermal growth factor pathway with a secretory fusion molecule. Cancer Res 2001; 61: 6377–6381.
Kanerva A, Mikheeva GV, Krasnykh V, Coolidge CJ, Lam JT, Mahasreshti PJ et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clin Cancer Res 2002; 8: 275–280.
Kanerva A, Wang M, Bauerschmitz GJ, Lam JT, Desmond RA, Bhoola SM et al. Gene transfer to ovarian cancer versus normal tissues with fiber-modified adenoviruses. Mol Ther 2002; 5: 695–704.
Grill J, Lamfers ML, van Beusechem VW, Dirven CM, Pherai DS, Kater M et al. The organotypic multicellular spheroid is a relevant three-dimensional model to study adenovirus replication and penetration in human tumors in vitro. Mol Ther 2002; 6: 609–614.
Lamfers ML, Grill J, Dirven CM, Van Beusechem VW, Geoerger B, Van Den Berg J et al. Potential of the conditionally replicative adenovirus Ad5-Delta24RGD in the treatment of malignant gliomas and its enhanced effect with radiotherapy. Cancer Res 2002; 62: 5736–5742.
Ryu R, Shin Y, Choi JW, Min W, Ryu H, Choi CR et al. Depletion of intracellular glutathione mediates zinc-induced cell death in rat primary astrocytes. Exp Brain Res 2002; 143: 257–263.
Coley HM, Lewandowicz G, Sargent JM, Verrill MW . Chemosensitivity testing of fresh and continuous tumor cell cultures using lactate dehydrogenase. Anticancer Res 1997; 17: 231–236.
Wood KV . Recent Advances and Prospects for Use of Beetle Luciferase as Genetic Reports. John Wiley and Sons; Chichester, 1991, 11p.
Acknowledgements
We thank Dr Robert S Negrin and Dr Christian Sheffold from the Division of Bone Marrow Transplantation at Stanford University Medical Center for use of the SK-OV-3luc cell line. This study was supported by NCI (R01 CA83821, P50 CA83591, P50 CA89019 and R01 CA93796), the Academy of Finland, Helsinki University Central Hospital Research Funds, University of Helsinki Internal Funds, Sohlberg Foundation, Sigrid Juselius Foundation, Finnish Cancer Society, Instrumentarium Research Fund, Emil Aaltonen Foundation, Finnish Oncology Association, Research and Science Foundation of Farmos.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lam, J., Hemminki, A., Kanerva, A. et al. A three-dimensional assay for measurement of viral-induced oncolysis. Cancer Gene Ther 14, 421–430 (2007). https://doi.org/10.1038/sj.cgt.7701028
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7701028
Keywords
This article is cited by
-
Isolation of more potent oncolytic paramyxovirus by bioselection
Gene Therapy (2013)
-
The chicken chorioallantoic membrane tumor assay as model for qualitative testing of oncolytic adenoviruses
Cancer Gene Therapy (2012)
-
In vitro spheroid model of placental vasculogenesis: does it work?
Laboratory Investigation (2009)