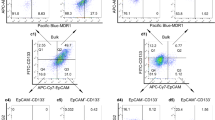
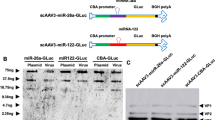
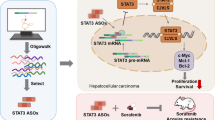
Abstract
Human cancers are characterized by a high degree of drug resistance. The multidrug resistance transporters MDR1-P-glycoprotein (ABCB1) and ABCC2 (MRP2) are expressed in a variety of human cancers, including hepatocellular carcinoma (HCC). The ABCC2 gene encodes a membrane protein involved in the ATP-dependent transport of conjugates of lipophilic substances. In this study we analyzed the effect of an ABCC2 antisense construct on the chemosensitization of HepG2 cells. Adenoviral vectors were constructed to allow an efficient expression of anti-ABCC2 antisense constructs. The effective target sequence comprised nucleotides 2543–2942 of the human ABCC2 cDNA. Adenoviral delivery of the ABCC2 antisense construct resulted in a reduced IC50 for doxorubicin (12-fold), vincristine (50-fold), cisplatin (25-fold) and etoposide (VP-16) (25-fold). The adenoviral delivery of the ABCC2 antisense construct was so efficient that chemosensitization of HepG2 cells could even be demonstrated in mass cell cultures without a selection of transduced cells for single ABCC2 antisense-expressing HCC cell clones. After transfection of the ABCC2 antisense-expressing construct, HepG2 cells had significantly reduced ABCC2 mRNA and ABCC2 protein levels. Transduction of the ABCC2 antisense-expressing construct into HepG2 cells resulted in the accumulation of the high-affinity ABCC2 substrate Fluo-3. HepG2 tumors stably transfected with an anti-ABCC2 antisense construct regressed significantly in nude mice upon vincristine treatment. In addition, significant tumor regression was also observed when adenovirus-expressing anti-ABCC2 antisense construct was directly injected into HepG2 tumors in nude mice. Our study demonstrates the specific reversal of ABCC2-related drug resistance in adenovirus-transduced HepG2 cells and in HepG2 tumors in nude mice expressing this ABCC2 antisense construct.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- ABCC2:
-
multidrug-related protein
- HCC:
-
hepatocellular carcinoma, p-gp, p-glycoprotein
- MDR:
-
multidrug resistance
References
Gottesman MM, Pastan I . Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem 1993; 62: 385–427.
Cole SP, Sparks KE, Fraser K, Loe DW, Grant CE, Wilson GM et al. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res 1994; 54: 5902–5910.
Cui Y, König J, Buchholz U, Spring H, Leier I, Keppler D . Drug resistance and ATP-dependent conjugate transport mediated by the apical multidrug resistance protein, ABCC2, permanently expressed in human and canine cells. Mol Pharmacol 1999; 55: 929–937.
Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA et al. Analysis of expression of cMOAT (ABCC2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 1997; 57: 3537–3547.
Koike K, Kawabe T, Tanaka T, Toh S, Uchiumi T, Wada M et al. (1997): A canalicular multispecific organic anion transporter (cMOAT) antisense DNA enhances drug sensitivity in human hepatic cancer cells. Cancer Res 1997; 57: 5475–5479.
Leslie EM, Deeley RG, Cole SP . Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol 2005; 204: 216–237.
Keppler D, Jedlitschky G, Leier I . Transport function and substrate specificity of multidrug resistance protein. Methods Enzymol 1998; 292: 607–616.
Tsuruo T, Iida H, Ohkochi E, Tsukagoshi S, Sakurai Y . Establishment and properties of vincristine-resistant human myelogenous leukemia K562. Jpn J Cancer Res 1983; 74: 751–758.
Teboekhorst PAW, Vankapel J, Schoester M, Sonneveld P . Reversal of typical multidrug resistance by cyclosporin and its non-immunosuppressive analog SDZ-PSC-833 in chinese hamster ovary cells expressing the mdr1-phenotype. Cancer Chemother Pharmacol 1992; 30: 238–242.
He TC, Zhou S, da Costa LT, Yu J, Kinzler KW, Vogelstein B . A simplified system for generating recombinant adenoviruses. Proc Natl Acad Sci USA 1998; 95: 2509–2514.
Park JG, Kramer BS, Steinberg SM, Carmichael J, Collins JM, Minna JD et al. Chemosensitivity testing of human colorectal carcinoma cell lines using a tetrazolium-based colorimetric assay. Cancer Res 1987; 47: 5875–5879.
Brown T, Mackey K . Analysis of RNA by Northern and slot blot hybridization. In: Chanda VB (ed). Current Protocols of Molecular Biology. John Wiley&Sons: New York, 1997 pp 4.9.1–4.9.14.
Ihrke G, Neufeld EB, Meads T, Shanks MR, Cassio D, Laurent M et al. WIF-B cells: an in vitro model for studies of hepatocyte polarity. J Cell Biol 1993; 123: 1761–1775.
Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM . Functional expression of human P-glycoprotein from plasmids using vaccinia virus-bacteriophage T7 RNA polymerase system. Methods Enzymol 1998; 292: 456–473.
Isshiki K, Nakao A, Ito M, Hamaguchi M, Takagi H . P-glycoprotein expression in human primary liver cancer and cirrhosis. J Hepatol 1993; 18: 168–172.
Cucco C, Calabretta B . In vitro and in vivo reversal of multidrug resistance in a human leukemia-resistant cell line by mdr1 antisense oligodeoxynucleotides. Cancer Res 1996; 56: 4332–4337.
Holm PS, Scanlon KJ, Dietel M . Reversion of multidrug resistance in the P-glycoprotein-positive human pancreatic cell line (EPP85-181RDB) by introduction of a hammerhead ribozyme. Br J Cancer 1994; 70: 239–243.
Masuda Y, Kobayashi H, Holland JF, Ohnuma T . Reversal of multidrug resistance by a liposome-MDR1 ribozyme complex. Cancer Chemother Pharmacol 1998; 42: 9–16.
Materna V, Liedert B, Thomale J, Lage H . Protection of platinum-DANN adduct formation and reversal of cisplatin resistance by anti-ABCC2 hammerhead ribozymes in human cancer cells. Int J Cancer 2005; 115: 393–402.
Itoh Y, Tamai M, Yokogawa K, Nomura M, Moritani S, Suzuki H et al. Involvement of multidrug resistance-associated protein 2 in in vivo cisplatin resistance of rat hepatoma AH66 cells. Anticancer Res 2002; 22: 1649–1653.
Kowalski P, Surowiak P, Lage H . Reversal of different drug-resistant phenotypes by an autocatalytic multitarget multiribozyme directed against the transcripts of the ABC transporters MDR1/P-gp, ABCC2; and BCRP. Mol Ther 2005; 11: 508–522.
Lischka K, Starke D, Failing K, Herling A, Kramer W, Petzinger E . Hepatobiliary elimination of bile acid-modified oligodeoxynucleotides in Wistar and TR-rats: evidence for ABCC2 as carrier for oligodeoxynucleotides. Biochem Pharmacol 2003; 66: 565–577.
Kaneko S, Hallenbeck P, Kotani T, Nakabayashi H, McGarrity G, Tamaoki T et al. Adenovirus-mediated gene therapy of hepatocellular carcinoma using cancer-specific gene expression. Cancer Res 1995; 55: 5283–5287.
Kanai F, Lan KH, Shiratori Y, Tanaka T, Ohashi M, Okudaira T et al. In vivo gene therapy for a-fetoprotein-producing hepatocellular carcinoma by adenovirus-mediated transfer of cytosine deaminase gene. Cancer Res 1997; 57: 461–465.
Goldstein LJ, Galski H, Fojo A, Willingham M, Lai SL, Gazdar A et al. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst 1989; 81: 116–124.
Li B, Gou XH, Chen L, Li DH, Zhao YH, Han L et al. Construction of the recombinant adenovirus vector carrying antisense multidrug resistance gene. Hepatobiliary Pancreat Dis Int 2006; 5: 80–84.
Cantz T, Nies AT, Brom M, Hofmann AF, Keppler D . ABCC2 a human conjugate export pump, is present and transports fluo 3 into apical vacuoles of Hep G2 cells. Am J Physiol Gastrointest Liver Physiol 2000; 278: G522–G531 80–84.
Acknowledgements
This work was supported by a grant from the Deutsche Forschungsgemeinschaft, Bonn, Germany (Ha2508/2-1, P H).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Folmer, Y., Schneider, M., Blum, H. et al. Reversal of drug resistance of hepatocellular carcinoma cells by adenoviral delivery of anti-ABCC2 antisense constructs. Cancer Gene Ther 14, 875–884 (2007). https://doi.org/10.1038/sj.cgt.7701082
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7701082
Keywords
This article is cited by
-
Establishment and characterization of an oxaliplatin-resistant hepatic cancer cell line*
Oncology and Translational Medicine (2018)
-
Effects of increased accumulation of doxorubicin due to emodin on efflux transporter and LRP1 expression in lung adenocarcinoma and colorectal carcinoma cells
Molecular and Cellular Biochemistry (2018)
-
Interferon-α sensitizes HBx-expressing hepatocarcinoma cells to chemotherapeutic drugs through inhibition of HBx-mediated NF-κB activation
Virology Journal (2013)
-
Adenoviral gene therapy in hepatocellular carcinoma: a review
Hepatology International (2013)
-
Lentivirus-mediated RNAi silencing targeting ABCC2 increasing the sensitivity of a human nasopharyngeal carcinoma cell line against cisplatin
Journal of Translational Medicine (2008)