Abstract
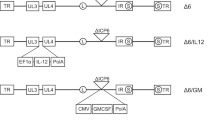
Herpes simplex viruses type 1 (HSV-1) that lack the γ134.5 gene are unable to replicate in the central nervous system (CNS), but maintain replication competence in actively dividing tumors. To determine if antitumor therapy by M002, a γ134.5− HSV that expresses interleukin-12 (IL-12), could be augmented by combinatorial therapy with another γ134.5-deleted HSV-1 engineered to express the chemokine CCL2, Neuro-2a tumors were established subcutaneously in the syngeneic A/J mouse strain. Tumors received multiple injections intratumorally either of saline, the parent, non-cytokine-expressing virus R3659, M002, M010 (γ134.5− HSV expressing CCL2), or a combination of M002 and M010. Efficacies were evaluated by monitoring inhibition of tumor growth over time. Results demonstrated the following: (1) inhibition of tumor growth was most pronounced in tumors treated with a combination of M002 and M010; (2) enhanced tumor growth inhibition for the combinatorial treatment group was statistically significant compared to either M002 or M010 alone; and (3) the variability between slopes of the tumor growth rates within an individual treatment group appeared to be virus-dependent, and was reproducible between experiments. Our results demonstrate that combinatorial cytokine/chemokine γ134.5− HSV therapies can provide superior antitumor effects in experimental tumors as a model for malignancies arising in the brain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Shapiro WR, Shapiro JR, Walker RW . Central nervous system. In: Abeloff MD, Armitage JO, Lichter AS et al, eds. Clinical Oncology. 2nd edn. New York, NY: Churchill Livingstone; 2000: 1103.
Markert JM, Gillespie GY, Weichselbaum RR, Roizman B, Whitley RJ . Genetically engineered HSV in the treatment of glioma: a review. Rev Med Virol. 2000;10:17–30.
Markert JM, Parker JN, Gillespie GY, Whitley RJ . Genetically engineered human herpes simplex virus in the treatment of brain tumours. Herpes. 2001;8:17–22.
Andreansky SS, He B, Gillespie GY, et al. The application of genetically engineered herpes simplex viruses to the treatment of experimental brain tumors. Proc Natl Acad Sci USA. 1996;93:11313–11318.
Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM . Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856.
Mineta T, Rabkin SD, Yazaki T, Hunter WD, Martuza RL . Attenuated multi-mutated herpes simplex virus-1 for the treatment of malignant gliomas. Nat Med. 1995;1:938–943.
Kramm CM, Chase M, Herrlinger U, et al. Therapeutic efficiency and safety of a second-generation replication-conditional HSV1 vector for brain tumor gene therapy. Hum Gene Ther. 1997;8:2057–2068.
Pyles RB, Warnick RE, Chalk CL, Szanti BE, Parysek LM . A novel multiply-mutated HSV-1 strain for the treatment of human brain tumors. Hum Gene Ther. 1997;8:533–544.
Markert JM, Malick A, Coen DM, Martuza RL . Reduction and elimination of encephalitis in an experimental glioma therapy model with attenuated herpes simplex mutants that retain susceptibility to acyclovir. Neurosurgery. 1993;32:597–603.
Chambers R, Gillespie GY, Soroceanu L, et al. Comparison of genetically engineered herpes simplex viruses for the treatment of brain tumors in a scid mouse model of human malignant glioma. Proc Natl Acad Sci USA. 1995;92:1411–1415.
Chou J, Kern ER, Whitley RJ, Roizman B . Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science. 1990;250:1262–1266.
Mineta T, Markert JM, Takamiya Y, et al. CNS tumor therapy by attenuated herpes simplex viruses. Gene Therapy. 1994;1(Suppl 1):S78.
Andreansky S, Soroceanu L, Flotte ER, et al. Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human malignant brain tumors. Cancer Res. 1997;57:1502–1509.
Andreansky S, He B, van Cott J, et al. Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. Gene Therapy. 1998;5:121–130.
Kaplitt MG, Tjuvajev JG, Leib DA, et al. Mutant herpes simplex virus induced regression of tumors growing in immunocompetent rats. J Neuro-Oncol. 1994;19:137–147.
Yazaki T, Manz HJ, Rabkin SD, Martuza RL . Treatment of human malignant meningiomas by G207, a replication-competent multimutated herpes simplex virus 1. Cancer Res. 1995;55:4752–4756.
Markert JM, Medlock MD, Rabkin SD, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Therapy. 2000;7:867–874.
Rampling R, Cruickshank G, Papanastassiou V, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Therapy. 2000;7:859–866.
Papanastassiou V, Rampling R, Fraser M, et al. The potential for efficacy of the modified (ICP 34.5(−)) herpes simplex virus HSV1716 following intratumoral injection into human malignant glioma: a proof of principle study. Gene Therapy. 2002;9:398–406.
Okada H, Giezeman-Smits KM, Tahara H, et al. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSVtk tumor vaccine. Gene Therapy. 1999;6:219–226.
Wei MX, Li F, Ono Y, Gauldie J, Chiocca EA . Effects on brain tumor cell proliferation by an adenovirus vector that bears the interleukin-4 gene. J Neurovirol. 1998;4:237–241.
Benedetti S, Dimeco F, Pollo B, et al. Limited efficacy of the HSV-TK/GCV system for gene therapy of malignant gliomas and perspectives for the combined transduction of the interleukin-4 gene. Hum Gene Ther. 1997;8:1345–1353.
Tseng SH, Hwang LH, Lin SM . Induction of antitumor immunity by intracerebrally implanted rat C6 glioma cells genetically engineered to secrete cytokines. J Immunother. 1997;20:334–342.
Nishimura T, Watanabe K, Yahata T, et al. The application of IL-12 to cytokine therapy and gene therapy for tumors. Ann NY Acad Sci. 1996;795:375–378.
Tahara H, Lotze MT . Antitumor effects of interleukin-12 (IL-12): applications for the immunotherapy and gene therapy of cancer. [Review] [32 refs]. Gene Therapy. 1995;2:96–106.
Lamont AG, Adorini L . IL-12: a key cytokine in immune regulation. Immunol Today. 1996;17:214–217.
Brunda MJ, Luistro L, Rumennik L, et al. Antitumor activity of interleukin 12 in preclinical models. Cancer Chemother Pharmacol. 1996;38:S16–S21.
Parker JN, Gillespie GY, Love CE, et al. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci USA. 2000;97:2208–2213.
Carr JA, Rogerson J, Mulqueen MJ, Roberts NA, Booth RFG . Interleukin-12 exhibits potent antiviral activity in experimental herpesvirus infections. J Virol. 1997;71:7799–7803.
Loetscher P, Seitz M, Clark-Lewis I, Baggiolini M, Moser B . Monocyte chemotactic proteins MCP-1, MCP-2, and MCP-3 are major attractants for human CD4+ and CD8+ T lymphocytes. FASEB J. 1994;8:1055–1060.
Traynor TR, Herring AC, Dorf ME, et al. Differential roles of CC chemokine ligand 2/monocyte chemotactic protein-1 and CCR2 in the development of T1 immunity. J Immunol. 2002;168:4659–4666.
Luther SA, Cyster JG . Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–107.
Post LE, Roizman B . A generalized technique for deletion of specific genes in large genomes: alpha gene 22 of herpes simplex virus 1 is not essential for growth. Cell. 1981;25:227–232.
Jenkins F, Roizman B . Herpes simplex virus 1 recombinants with noninverting genomes frozen in different isomeric arrangements are capable of independent replication. J Virol. 1986;59:494–499.
Lagunoff M, Roizman B . The regulation of synthesis and properties of the protein product of open reading frame P of the herpes simplex virus 1 genome. J Virol. 1995;69:3615–3623.
Lagunoff M, Roizman B . Expression of a herpes simplex virus 1 open reading frame antisense to the gamma134.5 gene and transcribed by an RNA 3′ coterminal with the unspliced latency-associated transcript. J Virol. 1994;68:6021–6028.
Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard EJ . Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989;142:1956–1962.
Lopez C . Genetics of natural resistance to herpesvirus infections in mice. Nature. 1975;258:152–153.
Macklis JD, Madison RD . Neuroblastoma grafts are noninvasively removed within mouse neocortex by selective laser activation of intracellular photolytic chromophore. J Neurosci. 1991;11:2055–2062.
Kikuchi T, Joki T, Saitoh S, et al. Anti-tumor activity of interleukin-2-producing tumor cells and recombinant interleukin 12 against mouse glioma cells located in the central nervous system. Int J Cancer. 1999;80:425–430.
Parney IF, Farr-Jones MA, Chang LJ, Petruk KC . Human glioma immunobiology in vitro: implications for immunogene therapy. Neurosurgery. 2000;46:1169–1177; discussion 1177–1178.
Dzenko KA, Andjelkovic AV, Kuziel WA, Pachter JS . The chemokine receptor CCR2 mediates the binding and internalization of monocyte chemoattractant protein-1 along brain microvessels. J Neurosci. 2001;21:9214–9223.
Sakai Y, Kaneko S, Nakamoto Y, et al. Enhanced anti-tumor effects of herpes simplex virus thymidine kinase/ganciclovir system by codelivering monocyte chemoattractant protein-1 in hepatocellular carcinoma. Cancer Gene Ther. 2001;8:695–704.
Tsuchiyama T, Kaneko S, Nakamoto Y, et al. Enhanced antitumor effects of a bicistronic adenovirus vector expressing both herpes simplex virus thymidine kinase and monocyte chemoattractant protein-1 against hepatocellular carcinoma. Cancer Gene Ther. 2003;10:260–269.
Russell SW, Gillespie GY . Nature, function and distribution of inflammatory cells in regressing and progressing Moloney sarcomas. J Reticuloendothelial Soc. 1977;22:159–168.
Gillespie GY, Hansen CB, Hoskins RG, Russell SW . Inflammatory cells in solid murine neoplasms. IV. Cytolytic T lymphocytes isolated from regressing or progressing Moloney sarcomas. J Immunol. 1977;119:564–570.
Gillespie GY, Russell SW . Level of activation determines whether inflammatory peritoneal and intratumoral macrophages will promote or suppress in vitro development of cytolytic T lymphocyte activity. J Reticuloendothelial Soc. 1980;27:535–545.
Gillespie GY, Barth RF . Cyclic variations in cell-mediated immunity to skin allografts detected by the technetium-99m microcytotoxicity assay. Cell Immunol. 1974;13:472–483.
Acknowledgements
We thank Dr Bernard Roizman, University of Chicago, for helpful discussion and comments throughout the course of this work. We thank Cammy Love, Karen Mardis and Sharon Samuel for excellent technical assistance with this work. Studies performed by the authors were initiated and supported in part by NCI P01 CA 71933 (RJW), by the Brain Tumor Society's Neal P Levitan Leadership Chair of Research Award (JNP), the NINDS Mentored Clinical Scientist Development Award (1K08NSO1942) (JMM), the American Association for Neurological Surgeons Young Investigator Award (JMM), the American Cancer Society Institutional Awards (JMM) and the American Brain Tumor Association (JMM).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Parker, J., Meleth, S., Hughes, K. et al. Enhanced inhibition of syngeneic murine tumors by combinatorial therapy with genetically engineered HSV-1 expressing CCL2 and IL-12. Cancer Gene Ther 12, 359–368 (2005). https://doi.org/10.1038/sj.cgt.7700784
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cgt.7700784
Keywords
This article is cited by
-
T-independent response mediated by oncolytic tanapoxvirus recombinants expressing interleukin-2 and monocyte chemoattractant protein-1 suppresses human triple negative breast tumors
Medical Oncology (2017)
-
Oncolytic tanapoxvirus expressing FliC causes regression of human colorectal cancer xenografts in nude mice
Journal of Experimental & Clinical Cancer Research (2015)
-
Bevacizumab With Angiostatin-armed oHSV Increases Antiangiogenesis and Decreases Bevacizumab-induced Invasion in U87 Glioma
Molecular Therapy (2012)
-
Engineered herpes simplex virus expressing bacterial cytosine deaminase for experimental therapy of brain tumors
Cancer Gene Therapy (2007)
-
Genetic strategies for brain tumor therapy
Cancer Gene Therapy (2006)