Abstract
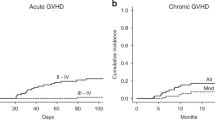
Anti-thymocyte globulin (ATG), raised in rabbits, is frequently used in allogeneic hematopoietic SCT (HSCT), to prevent graft rejection and acute GVHD. In solid organ transplant patients, antibodies to rabbit IgG result in an enhanced clearance of ATG. The occurrence of such antibodies in HSCT recipients and their clinical impact is unknown. Concentrations of ATG and anti-ATG antibodies were measured in 72 pediatric HSCT recipients treated with ATG as part of the conditioning. Anti-ATG antibodies were detected in 20 children (28%), all transplanted with a non-depleted graft. IgG anti-ATG, alone or combined with IgM and/or IgA anti-ATG, appeared in 10 children. Four patients developed IgG anti-ATG antibodies early (before day 22) post-HSCT. They had steep drops in ATG levels and showed rapid T-cell recovery, which was associated with a significantly increased risk of acute GVHD. In six patients IgG anti-ATG responses occurred later (range 28–46 days) after HSCT without an increased risk of GVHD. A total of 10 children only mounted an IgM (and IgA) anti-ATG response, which was without major impact on ATG levels. These results indicate that early development of IgG anti-ATG antibodies has a major impact on acute GVHD. Routine analysis ATG/anti-ATG Ab measurement should be considered.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ducloux D, Kazory A, Challier B, Coutet J, Bresson-Vautrin C, Motte G et al. Long-term toxicity of antithymocyte globulin induction may vary with choice of agent: a single-center retrospective study. Transplantation 2004; 77: 1029–1033.
Shapiro R, Young JB, Milford EL, Trotter JF, Bustami RT, Leichtman AB . Immunosuppression: evolution in practice and trends, 1993–2003. Am J Transplant 2005; 5: 874–886.
Ayuk F, Diyachenko G, Zabelina T, Wolschke C, Fehse B, Bacher U et al. Comparison of two doses of antithymocyte globulin in patients undergoing matched unrelated donor allogeneic stem cell transplantation. Biol Blood Marrow Transplant 2008; 14: 913–919.
Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di BP et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO). Blood 2001; 98: 2942–2947.
Remberger M, Mattsson J, Hausenberger D, Schaffer M, Svahn BM, Ringden O . Genomic tissue typing and optimal antithymocyte globuline dose using unrelated donors results in similar survival and relapse as HLA-identical siblings in haematopoietic stem-cell transplantation for leukaemia. Eur J Haematol 2008; 80: 419–428.
Scheinberg P, Wu CO, Nunez O, Young NS . Long-term outcome of pediatric patients with severe aplastic anemia treated with antithymocyte globulin and cyclosporine. J Pediatr 2008; 153: 814–819.
Bielory L, Wright R, Nienhuis AW, Young NS, Kaliner MA . Antithymocyte globulin hypersensitivity in bone marrow failure patients. JAMA 1988; 260: 3164–3167.
Prin MC, Renoult E, Kennel De MA, Bene MC, Kessler M, Faure GC . Serum anti-rabbit and anti-horse IgG, IgA, and IgM in kidney transplant recipients. Nephrol Dial Transplant 1997; 12: 2133–2139.
Book BK, Pescovitz MD, Agarwal A, Hardwick LL, Henson SL, Milgrom ML et al. In vitro monitoring of in vivo development of human anti-thymoglobulin antibodies by ELISA. Transplant Proc 2006; 38: 2869–2871.
Regan JF, Lyonnais C, Campbell K, Smith LV, Buelow R . Total and active thymoglobulin levels: effects of dose and sensitization on serum concentrations. Transpl Immunol 2001; 9: 29–36.
Regan JF, Campbell K, Van Smith L, Schroeder TJ, Womble D, Kano J et al. Sensitization following Thymoglobulin and Atgam rejection therapy as determined with a rapid enzyme-linked immunosorbent assay. US Thymoglobulin Multi-Center Study Group. Transpl Immunol 1999; 7: 115–121.
Bielory L, Gascon P, Lawley TJ, Nienhuis A, Frank MM, Young NS . Serum sickness and haematopoietic recovery with antithymocyte globulin in bone marrow failure patients. Br J Haematol 1986; 63: 729–736.
Schleuning M, Gunther W, Tischer J, Ledderose G, Kolb HJ . Dose-dependent effects of in vivo antithymocyte globulin during conditioning for allogeneic bone marrow transplantation from unrelated donors in patients with chronic phase CML. Bone Marrow Transplant 2003; 32: 243–250.
Waller EK, Langston AA, Lonial S, Cherry J, Somani J, Allen AJ et al. Pharmacokinetics and pharmacodynamics of anti-thymocyte globulin in recipients of partially HLA-matched blood hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant 2003; 9: 460–471.
Kakhniashvili I, Filicko J, Kraft WK, Flomenberg N . Heterogeneous clearance of antithymocyte globulin after CD34+-selected allogeneic hematopoietic progenitor cell transplantation. Biol Blood Marrow Transplant 2005; 11: 609–618.
Remberger M, Sundberg B . Rabbit-immunoglobulin G levels in patients receiving thymoglobulin as part of conditioning before unrelated donor stem cell transplantation. Haematologica 2005; 90: 931–938.
Eiermann TH, Lambrecht P, Zander AR . Monitoring anti-thymocyte globulin (ATG) in bone marrow recipients. Bone Marrow Transplant 1999; 23: 779–781.
Seidel MG, Fritsch G, Matthes-Martin S, Lawitschka A, Lion T, Potschger U et al. Antithymocyte globulin pharmacokinetics in pediatric patients after hematopoietic stem cell transplantation. J Pediatr Hematol Oncol 2005; 27: 532–536.
Call SK, Kasow KA, Barfield R, Madden R, Leung W, Horwitz E et al. Total and active rabbit antithymocyte globulin (rATG;Thymoglobulin) pharmacokinetics in pediatric patients undergoing unrelated donor bone marrow transplantation. Biol Blood Marrow Transplant 2009; 15: 274–278.
Meijer E, Bloem AC, Dekker AW, Verdonck LF . Effect of antithymocyte globulin on quantitative immune recovery and graft-versus-host disease after partially T-cell-depleted bone marrow transplantation: a comparison between recipients of matched related and matched unrelated donor grafts. Transplantation 2003; 75: 1910–1913.
Toor A, Rodriguez T, Bauml M, Mathews H, Shanti S, Senitzer D et al. Feasibility of conditioning with thymoglobulin and reduced intensity TBI to reduce acute GVHD in recipients of allogeneic SCT. Bone Marrow Transplant 2008; 42: 723–731.
Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J et al. Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day+7 after alternative donor transplants. Bone Marrow Transplant 2009; 45: 385–391.
Bacigalupo A . Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: efficacy and side effects. Bone Marrow Transplant 2005; 35: 225–231.
Basara N, Baurmann H, Kolbe K, Yaman A, Labopin M, Burchardt A et al. Antithymocyte globulin for the prevention of graft-versus-host disease after unrelated hematopoietic stem cell transplantation for acute myeloid leukemia: results from the multicenter German cooperative study group. Bone Marrow Transplant 2005; 35: 1011–1018.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 1995; 15: 825–828.
Rebello P, Cwynarski K, Varughese M, Eades A, Apperley JF, Hale G . Pharmacokinetics of CAMPATH-1 H in BMT patients. Cytotherapy 2001; 3: 261–267.
van Tol MJ, Gerritsen EJ, de Lange GG, van Leeuwen AM, Jol-van der Zijde CM, Oudeman-Gruber NJ et al. The origin of IgG production and homogeneous IgG components after allogeneic bone marrow transplantation. Blood 1996; 87: 818–826.
Hiemstra PS, Baldwin WM, van DV, Paul LC, van Es LA, Daha MR . Polymeric IgA antibody response to rabbit antithymocyte globulin in renal transplant recipients. Transplantation 1988; 45: 701–705.
Niblack G, Johnson K, Williams T, Green W, Richie R, MacDonell R . Antibody formation following administration of antilymphocyte serum. Transplant Proc 1987; 19: 1896–1897.
Pihusch R, Holler E, Muhlbayer D, Gohring P, Stotzer O, Pihusch M et al. The impact of antithymocyte globulin on short-term toxicity after allogeneic stem cell transplantation. Bone Marrow Transplant 2002; 30: 347–354.
Remberger M, Sundberg B . Low serum levels of total rabbit-IgG is associated with acute graft-versus-host disease after unrelated donor hematopoietic stem cell transplantation: results from a prospective study. Biol Blood Marrow Transplant 2009; 15: 996–999.
Labadie J, van Tol MJ, Dijkstra NH, van der KM, Jol-van der Zijde CM, de Lange GG et al. Transfer of specific immunity from donor to recipient of an allogeneic bone marrow graft: evidence for donor origin of the antibody producing cells. Br J Haematol 1992; 82: 437–444.
Storek J, Dawson MA, Lim LC, Burman BE, Stevens-Ayers T, Viganego F et al. Efficacy of donor vaccination before hematopoietic cell transplantation and recipient vaccination both before and early after transplantation. Bone Marrow Transplant 2004; 33: 337–346.
Wimperis JZ, Gottlieb D, Duncombe AS, Heslop HE, Prentice HG, Brenner MK . Requirements for the adoptive transfer of antibody responses to a priming antigen in man. J Immunol 1990; 144: 541–547.
Acknowledgements
We thank the medical and nursing staff of the pediatric transplant unit for implementing this study protocol, JLM Waaijer for her assistance in the management and sampling of patients, H Putter from the medical statistics department for advice, A van Strien for performing a literature study, and AGS van Halteren and MW Schilham for critically reading the manuscript.
Author contributions: CJvdZ designed and performed the study and prepared the manuscript, RB and MvT designed the study and prepared the manuscript, AJ-H and SR performed the measurements, RE and AL participated in interpretation of data and finalizing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Jol-van der Zijde, C., Bredius, R., Jansen-Hoogendijk, A. et al. IgG antibodies to ATG early after pediatric hematopoietic SCT increase the risk of acute GVHD. Bone Marrow Transplant 47, 360–368 (2012). https://doi.org/10.1038/bmt.2011.166
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2011.166
Keywords
This article is cited by
-
Successful mismatched hematopoietic stem cell transplantation for pediatric hemoglobinopathy by using ATG and post-transplant cyclophosphamide
Bone Marrow Transplantation (2021)
-
Population Pharmacokinetics of Alemtuzumab (Campath) in Pediatric Hematopoietic Cell Transplantation: Towards Individualized Dosing to Improve Outcome
Clinical Pharmacokinetics (2019)
-
Pharmacokinetics, Pharmacodynamics, and Pharmacogenomics of Immunosuppressants in Allogeneic Hematopoietic Cell Transplantation: Part II
Clinical Pharmacokinetics (2016)
-
Population Pharmacokinetic Modeling of Thymoglobulin® in Children Receiving Allogeneic-Hematopoietic Cell Transplantation: Towards Improved Survival Through Individualized Dosing
Clinical Pharmacokinetics (2015)
-
Antibodies to anti-thymocyte globulin in aplastic anemia patients have a negative impact on hematopoietic SCT
Bone Marrow Transplantation (2012)