Abstract
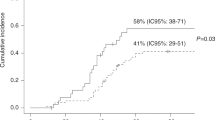
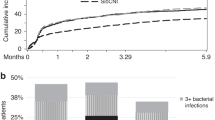
CMV can cause disease in several different organs after SCT. Seropositivity remains a major risk factor for TRM in unrelated SCT patients. In a study using the EBMT registry, CMV-seropositive patients receiving seropositive unrelated donor grafts had improved survival and reduced TRM compared with those receiving seronegative grafts, and a similar result was found in a single center study. Preventive measures can be divided into prevention of a primary infection or recurrence of CMV (prophylaxis) or prevention of development of disease when a reactivation has occurred (preemptive therapy). The standard therapy for CMV pneumonia has been i.v. ganciclovir combined with high-dose Ig, but this standard has never been evaluated in a controlled study and more recent studies have questioned whether the addition of Ig improves outcome.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Nichols WG, Corey L, Gooley T, Davis C, Boeckh M . High risk of death due to bacterial and fungal infection among cytomegalovirus (CMV)-seronegative recipients of stem cell transplants from seropositive donors: evidence for indirect effects of primary CMV infection. J Infect Dis 2002; 185: 273–282.
Ringden O, Schaffer M, Le Blanc K, Persson U, Hauzenberger D, Abedi MR et al. Which donor should be chosen for hematopoietic stem cell transplantation among unrelated HLA-A, -B, and -DRB1 genomically identical volunteers? Biol Blood Marrow Transplant 2004; 10: 128–134.
Boeckh M, Nichols WG . The impact of cytomegalovirus serostatus of donor and recipient before hematopoietic stem cell transplantation in the era of antiviral prophylaxis and preemptive therapy. Blood 2004; 103: 2003–2008.
Bowden RA, Slichter SJ, Sayers MH, Mori M, Cays MJ, Meyers JD . Use of leukocyte-depleted platelets and cytomegalovirus-seronegative red blood cells for prevention of primary cytomegalovirus infection after marrow transplant. Blood 1991; 78: 246–250.
Nichols WG, Price TH, Gooley T, Corey L, Boeckh M . Transfusion-transmitted cytomegalovirus infection after receipt of leukoreduced blood products. Blood 2003; 101: 4195–4200.
Ljungman P, Larsson K, Kumlien G, Aschan J, Barkholt L, Gustafsson-Jernberg A et al. Leukocyte depleted, unscreened blood products give a low risk for CMV infection and disease in CMV seronegative allogeneic stem cell transplant recipients with seronegative stem cell donors. Scand J Infect Dis 2002; 34: 347–350.
Bowden R, Cays M, Schoch G, Sayers M, Slichter S, Welk K et al. Comparison of filtered blood (FB) to seronegative blood products (SB) for prevention of cytomegalovirus (CMV) infection after marrow transplant. Blood 1995; 86: 3598–3603.
Prentice HG, Gluckman E, Powles RL, Ljungman P, Milpied N, Fernandez Ranada JM et al. Impact of long-term acyclovir on cytomegalovirus infection and survival after allogeneic bone marrow transplantation. European Acyclovir for CMV Prophylaxis Study Group. Lancet 1994; 343: 749–753.
Ljungman P, De La Camara R, Milpied N, Volin L, Russell CA, Webster A et al. A randomised study of valaciclovir as prophylaxis against CMV reactivation in allogeneic bone marrow transplant recipients. Blood 2002; 73: 930–936.
Winston DJ, Young JA, Pullarkat V, Papanicolaou GA, Vij R, Vance E et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem-cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood 2008.
Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood 2007; 110: 490–500.
Reusser P, Einsele H, Lee J, Volin L, Rovira M, Engelhard D et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood 2002; 99: 1159–1164.
Einsele H, Reusser P, Bornhauser M, Kalhs P, Ehninger G, Hebart H et al. Oral valganciclovir leads to higher exposure to ganciclovir than intravenous ganciclovir in patients following allogeneic stem cell transplantation. Blood 2006; 107: 3002–3008.
Nichols WG, Corey L, Gooley T, Drew WL, Miner R, Huang M et al. Rising pp65 antigenemia during preemptive anticytomegalovirus therapy after allogeneic hematopoietic stem cell transplantation: risk factors, correlation with DNA load, and outcomes. Blood 2001; 97: 867–874.
Gerna G, Lilleri D, Zecca M, Alessandrino EP, Baldanti F, Revello MG et al. Rising antigenemia levels may be misleading in pre-emptive therapy of human cytomegalovirus infection in allogeneic hematopoietic stem cell transplant recipients. Haematologica 2005; 90: 526–533.
Ljungman P, Deliliers GL, Platzbecker U, Matthes-Martin S, Bacigalupo A, Einsele H et al. Cidofovir for cytomegalovirus infection and disease in allogeneic stem cell transplant recipients. The Infectious Diseases Working Party of the European Group for Blood and Marrow Transplantation. Blood 2001; 97: 388–392.
Cesaro S, Zhou X, Manzardo C, Buonfrate D, Cusinato R, Tridello G et al. Cidofovir for cytomegalovirus reactivation in pediatric patients after hematopoietic stem cell transplantation. J Clin Virol 2005; 34: 129–132.
Kaptein SJ, Efferth T, Leis M, Rechter S, Auerochs S, Kalmer M et al. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res 2006; 69: 60–69.
Levi ME, Mandava N, Chan LK, Weinberg A, Olson JL . Treatment of multidrug-resistant cytomegalovirus retinitis with systemically administered leflunomide. Transpl Infect Dis 2006; 8: 38–43.
Ehlert K, Groll AH, Kuehn J, Vormoor J . Treatment of refractory CMV-infection following hematopoietic stem cell transplantation with the combination of foscarnet and leflunomide. Klin Padiatr 2006; 218: 180–184.
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101: 407–414.
Machado CM, Dulley FL, Boas LS, Castelli JB, Macedo MC, Silva RL et al. CMV pneumonia in allogeneic BMT recipients undergoing early treatment of pre-emptive ganciclovir therapy. Bone Marrow Transplant 2000; 26: 413–417.
Reusser P, Riddell SR, Meyers JD, Greenberg PD . Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 1991; 78: 1373–1380.
Krause H, Hebart H, Jahn G, Muller CA, Einsele H . Screening for CMV-specific T cell proliferation to identify patients at risk of developing late onset CMV disease. Bone Marrow Transplant 1997; 19: 1111–1116.
Ljungman P . Would monitoring CMV immune responses allow improved control of CMV in stem cell transplant patients. J Clin Virol 2006; 35: 493–495.
Gerna G, Lilleri D, Fornara C, Comolli G, Lozza L, Campana C et al. Monitoring of human cytomegalovirus-specific CD4 and CD8 T-cell immunity in patients receiving solid organ transplantation. Am J Transplant 2006; 6: 2356–2364.
Lilleri D, Fornara C, Furione M, Zavattoni M, Revello MG, Gerna G . Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J Infect Dis 2007; 195: 1062–1070.
Cwynarski K, Ainsworth J, Cobbold M, Wagner S, Mahendra P, Apperley J et al. Direct visualization of cytomegalovirus-specific T-cell reconstitution after allogeneic stem cell transplantation. Blood 2001; 97: 1232–1240.
Peggs K, Verfuerth S, Mackinnon S . Induction of cytomegalovirus (CMV)-specific T-cell responses using dendritic cells pulsed with CMV antigen: a novel culture system free of live CMV virions. Blood 2001; 97: 994–1000.
Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood 2002; 99: 3916–3922.
Szmania S, Galloway A, Bruorton M, Musk P, Aubert G, Arthur A et al. Isolation and expansion of cytomegalovirus-specific cytotoxic T lymphocytes to clinical scale from a single blood draw using dendritic cells and HLA-tetramers. Blood 2001; 98: 505–512.
Grigoleit GU, Kapp M, Hebart H, Fick K, Beck R, Jahn G et al. Dendritic cell vaccination in allogeneic stem cell recipients: induction of human cytomegalovirus (HCMV)-specific cytotoxic T lymphocyte responses even in patients receiving a transplant from an HCMV-seronegative donor. J Infect Dis 2007; 196: 699–704.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ljungman, P. CMV infections after hematopoietic stem cell transplantation. Bone Marrow Transplant 42 (Suppl 1), S70–S72 (2008). https://doi.org/10.1038/bmt.2008.120
Published:
Issue Date:
DOI: https://doi.org/10.1038/bmt.2008.120
Keywords
This article is cited by
-
Scoring system for clinically significant CMV infection in seropositive recipients following allogenic hematopoietic cell transplant: an SFGM-TC study
Bone Marrow Transplantation (2021)
-
Comparison of different cytomegalovirus diseases following haploidentical hematopoietic stem cell transplantation
Annals of Hematology (2020)
-
Combination of immunoglobulins and natural killer cells in the context of CMV and EBV infection
Medical Microbiology and Immunology (2014)
-
Monosomal karyotype predicts poor survival after allogeneic stem cell transplantation in chromosome 7 abnormal myelodysplastic syndrome and secondary acute myeloid leukemia
Leukemia (2013)