Abstract
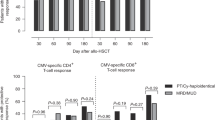
Patients experience cytomegalovirus (CMV) reactivation after stem cell transplantation (SCT) and need repeated courses of pre-emptive therapy. Analysis of CMV-specific immunity might help to assess the need for antiviral therapy. Forty-eight patients were studied during the first 3 months after SCT. Peripheral blood lymphocytes were stimulated by CMV antigen, and interferon (INF)-γ production by CD3+ and CD4+ T cells was analysed. Results were correlated to transplant factors and CMV disease. Patients with INF-γ production by CD3+ cells at 4 weeks after SCT had lower peak viral loads than patients with no such production (P=0.03). There was a similar tendency as regards CD4+ cells (P=0.09). Patients who underwent reduced-intensity conditioning (RIC) more frequently had CD3+ (48%) and CD4+ immunity (56%) 4 weeks after SCT compared with patients who received myeloablative conditioning (CD3+ 25%; CD4+ 35%). There was no effect of stem cell source, donor type or acute graft-versus-host disease. Three of 48 patients developed CMV disease and none of them had detectable INF-γ production. CMV-specific T-cell response is associated with a lower rate of CMV replication. RIC results in improved T-cell reconstitution. Recovery of CMV-specific immunity might be delayed in patients with CMV disease. These observations suggest that detection of CMV-specific T-cells is useful in assessing the immunity against CMV.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ljungman P, Engelhard D, Link H, Biron P, Brandt L, Brunet S et al. Treatment of interstitial pneumonitis due to cytomegalovirus with ganciclovir and intravenous immune globulin: experience of European Bone Marrow Transplant Group. Clin Infect Dis 1992; 14: 831–835.
Boeckh M, Bowden RA, Gooley T, Myerson D, Corey L . Successful modification of a pp65 antigenemia-based early treatment strategy for prevention of cytomegalovirus disease in allogeneic marrow transplant recipients. Blood 1999; 93: 1781–1782.
Hebart H, Einsele H . Clinical aspects of CMV infection after stem cell transplantation. Hum Immunol 2004; 65: 432–436.
Ljungman P . Immune reconstitution and viral infections after stem cell transplantation. Bone Marrow Transplant 1998; 21 (Suppl 2): S72–S74.
Hebart H, Einsele H . Specific infectious complications after stem cell transplantation. Support Care Cancer 2004; 12: 80–85.
Meyers JD, Flournoy N, Thomas ED . Risk factors for cytomegalovirus infection after human marrow transplantation. J Infect Dis 1986; 153: 478–488.
Lin TS, Zahrieh D, Weller E, Alyea EP, Antin JH, Soiffer RJ . Risk factors for cytomegalovirus reactivation after CD6+ T-cell-depleted allogeneic bone marrow transplantation. Transplantation 2002; 74: 49–54.
Patel SR, Ridwan RU, Ortin M . Cytomegalovirus reactivation in pediatric hemopoietic progenitors transplant: a retrospective study on the risk factors and the efficacy of treatment. J Pediatr Hematol Oncol 2005; 27: 411–415.
Stocchi R, Ward KN, Fanin R, Baccarani M, Apperley JF . Management of human cytomegalovirus infection and disease after allogeneic bone marrow transplantation. Haematologica 1999; 84: 71–79.
Ljungman P, Perez-Bercoff L, Jonsson J, Avetisyan G, Sparrelid E, Aschan J et al. Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica 2006; 91: 78–83.
Aubert G, Hassan-Walker AF, Madrigal JA, Emery VC, Morte C, Grace S et al. Cytomegalovirus-specific cellular immune responses and viremia in recipients of allogeneic stem cell transplants. J Infect Dis 2001; 184: 955–963.
Reusser P, Riddell SR, Meyers JD, Greenberg PD . Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood 1991; 78: 1373–1380.
Gratama JW, Kern F . Flow cytometric enumeration of antigen-specific T lymphocytes. Cytometry A 2004; 58: 79–86.
Le Blanc K, Remberger M, Uzunel M, Mattsson J, Barkholt L, Ringden O . A comparison of nonmyeloablative and reduced-intensity conditioning for allogeneic stem-cell transplantation. Transplantation 2004; 78: 1014–1020.
Rauser G, Einsele H, Sinzger C, Wernet D, Kuntz G, Assenmacher M et al. Rapid generation of combined CMV-specific CD4+ and CD8+ T-cell lines for adoptive transfer into recipients of allogeneic stem cell transplants. Blood 2004; 103: 3565–3572.
Ljungman P, Griffiths P, Paya C . Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 2002; 34: 1094–1097.
Reusser P, Einsele H, Lee J, Volin L, Rovira M, Engelhard D et al. Randomized multicenter trial of foscarnet versus ganciclovir for preemptive therapy of cytomegalovirus infection after allogeneic stem cell transplantation. Blood 2002; 99: 1159–1164.
Hakki M, Riddell SR, Storek J, Carter RA, Stevens-Ayers T, Sudour P et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood 2003; 102: 3060–3067.
Li CR, Greenberg PD, Gilbert MJ, Goodrich JM, Riddell SR . Recovery of HLA-restricted cytomegalovirus (CMV)-specific T-cell responses after allogeneic bone marrow transplant: correlation with CMV disease and effect of ganciclovir prophylaxis. Blood 1994; 83: 1971–1979.
Guillaume T, Rubinstein DB, Symann M . Immune reconstitution and immunotherapy after autologous hematopoietic stem cell transplantation. Blood 1998; 92: 1471–1490.
Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D et al. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood 2003; 101: 407–414.
Lilleri D, Gerna G, Fornara C, Lozza L, Maccario R, Locatelli F . Prospective simultaneous quantification of human cytomegalovirus-specific CD4+ and CD8+ T-cell reconstitution in young recipients of allogeneic hematopoietic stem cell transplantation. Blood 2006; 108: 1406–1412.
Ozdemir E, St John LS, Gillespie G, Rowland-Jones S, Champlin RE, Molldrem JJ et al. Cytomegalovirus reactivation following allogeneic stem cell transplantation is associated with the presence of dysfunctional antigen-specific CD8+ T cells. Blood 2002; 100: 3690–3697.
Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M et al. Immunologic recovery after hematopoietic cell transplantation with nonmyeloablative conditioning. Exp Hematol 2003; 31: 941–952.
Mohty M, Mohty AM, Blaise D, Faucher C, Bilger K, Isnardon D et al. Cytomegalovirus-specific immune recovery following allogeneic HLA-identical sibling transplantation with reduced-intensity preparative regimen. Bone Marrow Transplant 2004; 33: 839–846.
Ohnishi M, Sakurai T, Heike Y, Yamazaki R, Kanda Y, Takaue Y et al. Evaluation of cytomegalovirus-specific T-cell reconstitution in patients after various allogeneic haematopoietic stem cell transplantation using interferon-gamma-enzyme-linked immunospot and human leucocyte antigen tetramer assays with an immunodominant T-cell epitope. Br J Haematol 2005; 131: 472–479.
Lacey SF, Gallez-Hawkins G, Crooks M, Martinez J, Senitzer D, Forman SJ et al. Characterization of cytotoxic function of CMV-pp65-specific CD8+ T-lymphocytes identified by HLA tetramers in recipients and donors of stem-cell transplants. Transplantation 2002; 74: 722–732.
Gratama JW, van Esser JW, Lamers CH, Tournay C, Lowenberg B, Bolhuis RL et al. Tetramer-based quantification of cytomegalovirus (CMV)-specific CD8+ T lymphocytes in T-cell-depleted stem cell grafts and after transplantation may identify patients at risk for progressive CMV infection. Blood 2001; 98: 1358–1364.
Acknowledgements
We thank Hernán Concha Quezada for skilful technical support with flow cytometry. This study was supported by the Swedish Cancer Fund and research funds of the Karolinska Institutet. Gayane Avetisyan was the recipient of a scholarship from the Swedish Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Avetisyan, G., Larsson, K., Aschan, J. et al. Impact on the cytomegalovirus (CMV) viral load by CMV-specific T-cell immunity in recipients of allogeneic stem cell transplantation. Bone Marrow Transplant 38, 687–692 (2006). https://doi.org/10.1038/sj.bmt.1705507
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bmt.1705507
Keywords
This article is cited by
-
The Ten Most Common Questions on Cytomegalovirus Infection in Hematopoietic Stem Cell Transplant Patients
Clinical Hematology International (2022)
-
Reconstitution of CMV pp65 and IE-1-specific IFN-γ CD8+ and CD4+ T-cell responses affording protection from CMV DNAemia following allogeneic hematopoietic SCT
Bone Marrow Transplantation (2011)
-
The risk of early and late CMV DNAemia associated with Campath use in stem cell transplant recipients
Bone Marrow Transplantation (2010)
-
Functional analysis of cytomegalovirus-specific T lymphocytes compared to tetramer assay in patients undergoing hematopoietic stem cell transplantation
Bone Marrow Transplantation (2008)
-
Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT
Bone Marrow Transplantation (2007)