Abstract
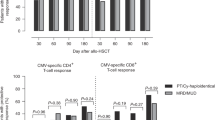
Prophylactic infusion of selected donor T cells can be an effective method to restore specific immunity after T-cell-depleted allogeneic stem cell transplantation (TCD-alloSCT). In this phase I/II study, we aimed to reduce the risk of viral complications and disease relapses by administrating donor-derived CD8pos T cells directed against cytomegalovirus (CMV), Epstein-Barr virus (EBV) and adenovirus antigens, tumor-associated antigens (TAA) and minor histocompatibility antigens (MiHA). Twenty-seven of thirty-six screened HLA-A*02:01pos patients and their CMVpos and/or EBVpos donors were included. Using MHC-I-Streptamers, 27 T-cell products were generated containing a median of 5.2 × 106 cells. Twenty-four products were administered without infusion-related complications at a median of 58 days post alloSCT. No patients developed graft-versus-host disease during follow-up. Five patients showed disease progression without coinciding expansion of TAA/MiHA-specific T cells. Eight patients experienced CMV- and/or EBV-reactivations. Four of these reactivations were clinically relevant requiring antiviral treatment, of which two progressed to viral disease. All resolved ultimately. In 2/4 patients with EBV-reactivations and 6/8 patients with CMV-reactivations, viral loads were followed by the expansion of donor-derived virus target-antigen-specific T cells. In conclusion, generation of multi-antigen-specific T-cell products was feasible, infusions were well tolerated and expansion of target-antigen-specific T cells coinciding viral reactivations was illustrated in the majority of patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Thomas ED. Bone marrow transplantation from bench to bedside. Ann N Y Acad Sci. 1995;770:34–41.
Kolb HJ. Graft-versus-leukemia effects of transplantation and donor lymphocytes. Blood. 2008;112:4371–83.
Falkenburg JH, Warren EH. Graft versus leukemia reactivity after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:S33–8.
Urbano-Ispizua A, Garcia-Conde J, Brunet S, Hernandez F, Sanz G, Petit J, et al. High incidence of chronic graft versus host disease after allogeneic peripheral blood progenitor cell transplantation. the Spanish Group of Allo-PBPCT. Haematologica. 1997;82:683–9.
Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–52.
Martin PJ, Hansen JA, Buckner CD, Sanders JE, Deeg HJ, Stewart P, et al. Effects of in vitro depletion of T cells in HLA-identical allogeneic marrow grafts. Blood. 1985;66:664–72.
Hale G. The CD52 antigen and development of the CAMPATH antibodies. Cytotherapy. 2001;3:137–43.
Chakrabarti S, MacDonald D, Hale G, Holder K, Turner V, Czarnecka H, et al. T-cell depletion with Campath-1H “in the bag” for matched related allogeneic peripheral blood stem cell transplantation is associated with reduced graft-versus-host disease, rapid immune constitution and improved survival. Br J Haematol. 2003;121:109–18.
Barge RM, Starrenburg CW, Falkenburg JH, Fibbe WE, Marijt EW, Willemze R. Long-term follow-up of myeloablative allogeneic stem cell transplantation using Campath “in the bag” as T-cell depletion: the Leiden experience. Bone Marrow Transpl. 2006;37:1129–34.
Howard DS, Phillips IG, Reece DE, Munn RK, Henslee-Downey J, Pittard M, et al. Adenovirus infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 1999;29:1494–501.
La Rosa AM, Champlin RE, Mirza N, Gajewski J, Giralt S, Rolston KV, et al. Adenovirus infections in adult recipients of blood and marrow transplants. Clin Infect Dis. 2001;32:871–6.
Styczynski J, Einsele H, Gil L, Ljungman P. Outcome of treatment of Epstein-Barr virus-related post-transplant lymphoproliferative disorder in hematopoietic stem cell recipients: a comprehensive review of reported cases. Transpl Infect Dis. 2009;11:383–92.
Ljungman P, Hakki M, Boeckh M. Cytomegalovirus in hematopoietic stem cell transplant recipients. Hematol Oncol Clin North Am. 2011;25:151–69.
Reusser P, Riddell SR, Meyers JD, Greenberg PD. Cytotoxic T-lymphocyte response to cytomegalovirus after human allogeneic bone marrow transplantation: pattern of recovery and correlation with cytomegalovirus infection and disease. Blood. 1991;78:1373–80.
Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, et al. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44.
Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, et al. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–22.
Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–7.
Peggs KS, Mackinnon S. Augmentation of virus-specific immunity after hematopoietic stem cell transplantation by adoptive T-cell therapy. Hum Immunol. 2004;65:550–7.
Feuchtinger T, Matthes-Martin S, Richard C, Lion T, Fuhrer M, Hamprecht K, et al. Safe adoptive transfer of virus-specific T-cell immunity for the treatment of systemic adenovirus infection after allogeneic stem cell transplantation. Br J Haematol. 2006;134:64–76.
Feuchtinger T, Opherk K, Bethge WA, Topp MS, Schuster FR, Weissinger EM, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010;116:4360–7.
Heslop HE, Slobod KS, Pule MA, Hale GA, Rousseau A, Smith CA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115:925–35.
Schmitt A, Tonn T, Busch DH, Grigoleit GU, Einsele H, Odendahl M, et al. Adoptive transfer and selective reconstitution of streptamer-selected cytomegalovirus-specific CD8+ T cells leads to virus clearance in patients after allogeneic peripheral blood stem cell transplantation. Transfusion. 2011;51:591–9.
Meij P, Jedema I, Zandvliet ML, van der Heiden PL, van de Meent M, van Egmond HM, et al. Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8+ T-cell lines. J Immunother. 2012;35:621–8.
Neuenhahn M, Albrecht J, Odendahl M, Schlott F, Dossinger G, Schiemann M, et al. Transfer of minimally manipulated CMV-specific T cells from stem cell or third-party donors to treat CMV infection after allo-HSCT. Leukemia. 2017;31:2161–71.
Jacobsen T, Sifontis N. Drug interactions and toxicities associated with the antiviral management of cytomegalovirus infection. Am J Health Syst Pharm. 2010;67:1417–25.
Kapp M, Stevanovic S, Fick K, Tan SM, Loeffler J, Opitz A, et al. CD8+ T-cell responses to tumor-associated antigens correlate with superior relapse-free survival after allo-SCT. Bone Marrow Transpl. 2009;43:399–410.
Anguille S, Van Tendeloo VF, Berneman ZN. Leukemia-associated antigens and their relevance to the immunotherapy of acute myeloid leukemia. Leukemia. 2012;26:2186–96.
Griffioen M, van Bergen CA, Falkenburg JH. Autosomal minor histocompatibility antigens: how genetic variants create diversity in immune targets. Front Immunol. 2016;7:100.
Marijt WA, Heemskerk MH, Kloosterboer FM, Goulmy E, Kester MG, van der Hoorn MA, et al. Hematopoiesis-restricted minor histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remissions of relapsed leukemia. Proc Natl Acad Sci USA. 2003;100:2742–7.
Micklethwaite KP, Clancy L, Sandher U, Hansen AM, Blyth E, Antonenas V, et al. Prophylactic infusion of cytomegalovirus-specific cytotoxic T lymphocytes stimulated with Ad5f35pp65 gene-modified dendritic cells after allogeneic hemopoietic stem cell transplantation. Blood. 2008;112:3974–81.
Mackinnon S, Thomson K, Verfuerth S, Peggs K, Lowdell M. Adoptive cellular therapy for cytomegalovirus infection following allogeneic stem cell transplantation using virus-specific T cells. Blood Cells Mol Dis. 2008;40:63–7.
Leen AM, Christin A, Myers GD, Liu H, Cruz CR, Hanley PJ, et al. Cytotoxic T lymphocyte therapy with donor T cells prevents and treats adenovirus and Epstein-Barr virus infections after haploidentical and matched unrelated stem cell transplantation. Blood. 2009;114:4283–92.
Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood. 2016;127:3331–40.
Houghtelin A, Bollard CM. Virus-Specific T cells for the immunocompromised patient. Front Immunol. 2017;8:1272.
Morris EC, Rebello P, Thomson KJ, Peggs KS, Kyriakou C, Goldstone AH, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–6.
Freimuller C, Stemberger J, Artwohl M, Germeroth L, Witt V, Fischer G, et al. Selection of adenovirus-specific and Epstein-Barr virus-specific T cells with major histocompatibility class I streptamers under Good Manufacturing Practice (GMP)-compliant conditions. Cytotherapy. 2015;17:989–1007.
Roex MCJ, Hageman L, Heemskerk MT, Veld SAJ, van Liempt E, Kester MGD, et al. The simultaneous isolation of multiple high and low frequent T-cell populations from donor peripheral blood mononuclear cells using the major histocompatibility complex I-Streptamer isolation technology. Cytotherapy. 2018;20:543–55.
Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–86.
Stemberger C, Graef P, Odendahl M, Albrecht J, Dossinger G, Anderl F, et al. Lowest numbers of primary CD8(+) T cells can reconstitute protective immunity upon adoptive immunotherapy. Blood. 2014;124:628–37.
Loeff FC, van Egmond EHM, Moes D, Wijnands C, Von Dem Borne PA, Veelken H, et al. Impact of alemtuzumab pharmacokinetics on T-cell dynamics, graft-versus-host disease and viral reactivation in patients receiving allogeneic stem cell transplantation with an alemtuzumab-based T-cell-depleted graft. Transpl Immunol. 2019. (In press).
Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18:295–304.
Chalandon Y, Passweg JR, Schmid C, Olavarria E, Dazzi F, Simula MP, et al. Outcome of patients developing GVHD after DLI given to treat CML relapse: a study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transpl. 2010;45:558–64.
Chakrabarti S, Mackinnon S, Chopra R, Kottaridis PD, Peggs K, O'Gorman P, et al. High incidence of cytomegalovirus infection after nonmyeloablative stem cell transplantation: potential role of Campath-1H in delaying immune reconstitution. Blood. 2002;99:4357–63.
Seggewiss R, Einsele H. Immune reconstitution after allogeneic transplantation and expanding options for immunomodulation: an update. Blood. 2010;115:3861–8.
Leen AM, Bollard CM, Myers GD, Rooney CM. Adenoviral infections in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:243–51.
Rouce RH, Louis CU, Heslop HE. Epstein-Barr virus lymphoproliferative disease after hematopoietic stem cell transplant. Curr Opin Hematol. 2014;21:476–81.
van der Heiden P, Marijt E, Falkenburg F, Jedema I. Control of cytomegalovirus viremia after allogeneic stem cell transplantation: a review on CMV-specific T cell reconstitution. Biology of blood and marrow transplantation. J Am Soc Blood aMarrow Transplant. 2018;24:1776–82.
van der Heiden PLJ, van Egmond HM, Veld SAJ, van de Meent M, Eefting M, de Wreede LC, et al. CMV seronegative donors: effect on clinical severity of CMV infection and reconstitution of CMV-specific immunity. Transpl Immunol. 2018;49:54–8.
Acknowledgements
The authors thank patients, donors and medical staff involved in this clinical trial; Lisette Bogers and Susan Collins for excellent managing of clinical study data; Employees of the Laboratory for Stem Cell Therapy, Laboratory for Specialized Hematology and the Interdivisional GMP Facility of the LUMC for logistical, technical and GMP assistance. This study was financially supported by the European Union’s seventh Framework Program (FP/2007–2013) under grant agreement number 601722 and by Dutch Cancer Society grant UL 2008-4263.
Author information
Authors and Affiliations
Contributions
JHFF, IJ, CJMH, LCW, SD, and HE developed the clinical study concept; CJMH, PB, JHFF, HV, and MRS enrolled study participants and monitored patients clinically; EL, IJ, EE, and SAJV wrote the protocols for multi-antigen-specific T-cell product generation; JJZ was responsible for donor leukapheresis logistics and release; LG provided MHC-I-Streptamer technology equipment; PM was involved in GMP logistics and product release; LH, EE, SAJV, CH,EL, and MCJR generated multi-antigen-specific T-cell products and performed in-depth analysis of the T-cell products; PB, CJMH, and MCJR administered the T-cell products to the patients; LH, SAJV, MCJR, ACTMV, and IJ performed laboratory monitoring; PB, JHFF, CJMH, IJ, and MCJR collected clinical/laboratory follow-up data and performed analysis; LCW assisted with statistical analysis; MCJR, IJ, and JHFF wrote the manuscript; all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
LG is senior vice president and managing director of Juno Therapeutics GmbH, a Celgene company (Munich, Germany). The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Roex, M.C.J., van Balen, P., Germeroth, L. et al. Generation and infusion of multi-antigen-specific T cells to prevent complications early after T-cell depleted allogeneic stem cell transplantation—a phase I/II study. Leukemia 34, 831–844 (2020). https://doi.org/10.1038/s41375-019-0600-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-019-0600-z
This article is cited by
-
Persistence of ex vivo expanded tumour and pathogen specific T-cells after allogeneic stem cell transplant for myeloid malignancies (the INTACT study)
Leukemia (2023)
-
T-cell receptor repertoire of cytomegalovirus-specific cytotoxic T-cells after allogeneic stem cell transplantation
Scientific Reports (2020)