Key Points
-
Lasers can be used in the surgical management of gingival hyperplasias, tooth exposure and hyperpigmentation.
-
Precise excision of gingival tissue relative to restorative procedures, with associated haemostatic control, can often allow treatment to proceed more smoothly and quickly.
-
Care should be observed when using lasers in areas where there is a close approximation of various hard and soft tissues, to avoid unwanted thermal damage.
Key Points
Lasers in dentistry
-
1
Introduction, history of lasers and laser light production
-
2
Laser-tissue interaction
-
3
Low-level laser use in dentistry
-
4
Lasers and soft tissue: 'loose' soft tissue surgery
-
5
Lasers and soft tissue: 'fixed' soft tissue surgery
-
6
Lasers and soft tissue: periodontal therapy
-
7
Surgical laser use in implantology and endodontics
-
8
Surgical lasers and hard dental tissue
-
9
Laser regulation and safety in general dental practice
Abstract
Within a general practice setting, there are few benign pathological conditions of the attached or keratinised gingival complex that are not amenable to simple surgical intervention. The majority of surgical procedures are adjunctive to the delivery of restorative dentistry. There is an understandable dogma worldwide towards the management of soft tissues as they interface with restorative procedures. Contemporary teaching, both at undergraduate and postgraduate level, would recognise the need for a period of wound healing and stability, based on scalpel-induced incisional therapy. The use of laser wavelengths, based on predictable evidence-based protocols, has re-defined the surgical management of keratinised mucosa that is bound to the underlying periosteum and bone. This can be seen as being of benefit to the clinician in determining the outcome, and the patient in achieving quality results.
Similar content being viewed by others
Surgical procedures
The range of benign pathology affecting the muco-periosteal tissue of the dento-alveolar complex includes the following:
-
Epulis
-
Giant cell granuloma
-
Inflammatory and drug-induced gingival hyperplasia
-
Tooth exposure
-
Melanin removal.
In addition, there is a range of gingival adaptation procedures, both to allow restorative procedures and to allow access to restorative margins during restorative procedures.
When used correctly, laser energy will act primarily as a means of incision, excision or ablation. Its advantage over the scalpel is the avoidance of bleeding, dressings and sutures and lowered potential for post-operative bacterial contamination. The use of a laser should neither supplant nor compromise a sound approach to surgical technique or correct patient management. Wherever appropriate, laser surgery in and around the gingival cuff should seek to preserve a biological width, ie the zones of connective and epithelial tissues attached to the tooth, minimum 3 mm in depth, that maintains alveolar bone height, gingival margin stability and health and prevents overgrowth.1,2,3
Laser-tissue interaction and keratinised mucosa – risk analysis
As discussed before, the prime interactive mechanism is photothermal, ie incident laser energy is absorbed by target chromophores and converted to heat, which effects tissue change. With shorter wavelengths (diode, Nd:YAG), there is risk of deeper penetration of the light energy. With longer wavelengths (Er,Cr:YSGG, Er:YAG and CO2), tissue penetration is considerably less, but there is potential for the build-up of char (carbonised products of ablation). Keratinised mucosa overlying the alveolar ridge exists as an outer epithelium layer and an inner connective tissue layer, separated by the basal lamina, in thickness from 0.5-4.0 mm.4 The underlying periosteum and bone, together with root surface at gingival margin levels, can be susceptible to thermal damage. This risk can exist through the penetration of short wavelengths or the conductive heat effects arising from long wavelength char that is super-heated.5,6,7,8,9
It is advisable, therefore, to assess the thickness, vascularity and position of any target gingival tissue, together with an appraisal of adjacent bone and tooth tissue.
Post-ablation tissue should be discarded, either through the use of a curette or damp cotton wool or gauze, to minimise the build-up of carbonised debris.
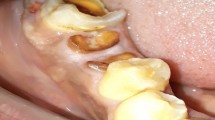
Figures 1,2,3,4 demonstrate the risk of deep penetration of a near-infrared Nd:YAG laser wavelength6,10,11,12 during the removal of a giant cell granuloma. Laser therapy has been cited as a possible modality in treating this condition.13 A perceived need to remove all granulomatous tissue resulted in the (erroneous) choice of excessive power values (>5.0 Watts average power). Not only did it become evident through uncharacteristic post-operative pain that too much power had been used, but also, within a few weeks, a bone sequestrum appeared. In addition, within a year, the tooth required root-canal therapy, possibly due to transmitted laser energy. Thankfully, the patient accepted the consequences with good grace and the soft tissue complex regained its integrity and stability through the ensuing 10 years.
A comparison of laser-tissue interaction between two differing wavelengths, illustrating expected and unwanted effects, is shown in Figures 5,6,7,8,9,10,11,12,13. This involved the removal of free-gingival tissue, commensurate with biological width, prior to placement of new crowns at UL1, UR1. The increased vascularity of the left gingival tissue prompted the use of a pigment-absorbed laser wavelength14 (diode 810 nm), whereas the right gingival tissue, of a more normal consistency, merited the use of a CO2 wavelength.15
As is often seen, the use of near-infrared wavelength laser energy results in desiccation and fragmentation of tissue, which can be removed with a sharp curette. Comparatively, the longer CO2 laser, used in a slightly non-contact mode, gives rise to a wider zone of ablation and a less accurate incision line due to beam divergence.16 In this case, it is evident that the tooth tissue has been exposed to the laser energy, resulting in a 'white area' of surface interaction.17 The tooth would be subsequently prepared for restoration and the disrupted tooth surface removed.
In order to protect the adjacent, or underlying, tooth tissue, a non-reflective, non absorbent instrument can be positioned within the gingival sulcus.18
Laser excision of benign pathology
To perform a laser procedure properly, the surgeon uses the laser with the correct wavelength and selects the appropriate fluence and exposure time to achieve a selective photothermolytic, photomechanical or photochemical effect on the target. In earlier articles in this series, the assessment of average power was explained and, with regard to the photothermolytic interaction, due regard should be given to thermal relaxation in order to minimise unwanted effects. Laser power levels of 1.5-3.0 Watts with intervals are considered sufficient for most, if not all gingival procedures. With free-running pulsed (FRP) deliveries, shorter pulse-widths will produce higher peak values and ablate faster, but risk collateral damage; higher numbers of pulses per second, even coupled with low energy-per-pulse values, will result in less thermal relaxation. It is considered good practice with all wavelengths to select a low energy level at first, or to approach the tissue in a de-focussed mode, to assess the level of interaction.
With simple hyperplasia, wherever possible, the laser beam should be directed into the discard tissue and excision completed in a careful and deliberate manner (Figs 14,15,16,17).
With a more pedunculated epulis, it should be possible to aid excision by placing the lesion under tension. Aetiological factors should obviously be addressed to prevent recurrence (Figs 18,19,20,21).
The use of lasers to treat drug-induced gingival hyperplasia has been advocated through several studies. This can be of great assistance where either the general medical condition merits a simple surgical procedure, or the underlying blood-picture is compromised.19,20,21,22,23 The associated cervical caries arising from limited access can be treated concurrently, due to the ability to control soft tissue bleeding (Figs 22,23,24,25).
The facility to combine soft tissue management with hard tissue treatment is a major benefit of laser use, when compared to more conventional therapy. Not only does this represent considerable benefit to the practitioner, but the patient management is deemed less complicated, as appointments can be condensed and sutures and dressing packs avoided. Very often, a tooth fracture, otherwise committed to extraction, can be treated and restored successfully, resulting in many more years of function. The often-achieved phenomenon of stability of the post-surgical gingival margin can be utilised in achieving good aesthetic results (Figs 26,27,28,29).
Where the fracture has extended below the alveolar bone margin, or where the biologic width might be compromised, the safe ablation of bone with either Er:YAG or Er,Cr:YSGG lasers can be carried out to allow correct exposure of the fracture margin (Figs 30,31,32,33,34,35).
In the surgical adjunct to orthodontics, from gingival hyperplasia associated with orthodontic appliances, to the exposure of un-erupted teeth, the use of laser wavelengths can often enable simple procedures to be carried out without subjecting the child patient to additional anxiety24,25,26 (Figs 36,37,38,39). Both short and long wavelengths can be used, taking care not to cause damage to the underlying tooth or bone, relative to wavelength. The control of bleeding will allow the placement of bonded brackets, without undue risk of failure.
Where aesthetics are compromised by melanin patches within the attached gingiva, often seen in Asian and African ethnic groups, an alternative approach to a 'dermabrasion' technique is to use laser energy (Figs 40,41,42,43). Most current wavelengths have been advocated, citing either selective pigment ablation with short wavelengths or superficial layer ablation of the tissue with longer wavelengths.27,30,31 The correct use of the selected laser results in little or no discomfort or inflammation, compared to removal using rotary instrumentation.
Conclusion
The development of various laser systems in dentistry during the past 15 years has enabled a range of soft tissue procedures to be carried out. The benefits of haemostasis, sterility, reduced discomfort and obviation of dressings, merit advantages for both clinician and patient. The initial anecdotal reports of success that for many years sought to underline the credibility of laser use have been investigated through in vitro and in vivo studies. What is in little doubt is the predictability of laser use, based upon the biophysics of laser-tissue interaction; such an evidence-based approach continues to allow this treatment modality to expand its application in surgical dentistry.
References
Lanning S K, Waldrop T C, Gunsolley J C, Maynard J G . Surgical crown lengthening: evaluation of the biological width. J Periodontol 2003; 74: 468–474.
Gracis S, Fradeani M, Celletti R, Bracchetti G . Biological integration of aesthetic restorations: factors influencing appearance and long-term success. Periodontol 2000 2001; 27: 29–44.
Adams T C, Pang P K . Lasers in aesthetic dentistry. Dent Clin North Am 2004; 48: 833–860, vi.
Kydd W L, Daly C H, Wheeler J B 3rd . The thickness measurements of masticatory mucosa in vivo. Int Dent J 1971; 21: 430–441.
Pogrel M A, McCracken K J, Daniels T E . Histologic evaluation of the width of soft tissue necrosis adjacent to carbon dioxide laser incisions. Oral Surg Oral Med Oral Pathol 1990; 70: 564–568.
Spencer P, Cobb C M, Wieliczka D M, Glaros A G, Morris P J . Change in temperature of subjacent bone during soft tissue laser ablation. J Periodontol 1998; 69: 1278–1282.
Hall R R . The healing of tissues incised by a carbon dioxide laser. Br J Surg 1971; 58: 222–225.
Fisher S E, Frame J W . The effects of the carbon dioxide surgical laser on oral tissues. Br J Oral Maxillofac Surg 1984; 22: 414–425.
Pick R M, Pecaro B C . Use of the CO2 laser in soft tissue dental surgery. Lasers Surg Med 1987; 7: 207–213.
Wilder-Smith P, Arrastia A M, Schell M J, Grill G, Berns M W . Effect of Nd:YAG laser irradiation and root planning on the root surface: structural and thermal effects. J Periodontol 1995; 66: 1032–1039.
White J M, Fagan M C, Goodis H E . ntra-pulpal temperatures during pulsed Nd:YAG laser treatment of dentin in vitro. J Periodontol 1994; 65: 255–259.
Tokita Y, Sunakawa M, Suda H . Pulsed Nd:YAG laser irradiation of the tooth pulp in the cat: I. Effect of spot lasing. Lasers Surg Med 2000; 26: 398–404.
Chaparro-Avendano A V, Berini-Aytes L, Gay-Escoda C . Peripheral giant cell granuloma. A report of five cases and review of the literature. Med Oral Patol Oral Cir Bucal 2005; 10: 53–57; 48–52.
Wyman A, Duffy S, Sweetland H M, Sharp F, Rogers K . Preliminary evaluation of a new high power diode laser. Lasers Surg Med 1992; 12: 506–509.
Hall R R, Hill D W, Beach A D . A carbon dioxide surgical laser. Ann Royal Coll Surg Eng 1971; 48: 181–188.
Halldorsson T, Langerholc J . Thermodynamic analysis of laser irradiation of biological tissue. Appl Opt 1978; 17: 3948–3958.
Goultschin J, Gazil D, Bichacho N, Bab I . Changes in teeth and gingiva of dogs following laser surgery: A block surface light microscope study. Lasers Surg Med 1988; 8: 402–408.
Powell G L, Whisenant B K, Morton T H . Carbon dioxide laser oral safety parameters for teeth. Lasers Surg Med 1990; 10: 389–392.
Barak S, Kaplan I . The CO2 laser in the excision of gingival hyperplasia caused by nifedipine. J Clin Periodontol 1988; 15: 633–635.
Roed-Petersen B . The potential use of CO2-laser gingivectomy for phenytoin-induced gingival hyperplasia in mentally retarded patients. J Clin Periodontol 1993; 20: 729–731.
Hylton R P . Use of CO2 laser for gingivectomy in a patient with Sturge-Weber disease complicated by dilantin hyperplasia. J Oral Maxillofac Surg 1986; 44: 646–648.
Rossmann J A, Ingles E, Brown R S . Multi-modal treatment of drug-induced gingival hyperplasia in a kidney transplant patient. Compend Contin Educ Dent 1994; 15: 1266–1276.
Hattler A B, Kirschner R A, Susanin P B . Laser surgery for immunosuppressive gingival hyperplasia. Periodontal Clin Investig 1992; 14: 18–20.
Parkins F M, Miller R L, Furnish G M, O'Toole T J . A preliminary report: YAG laser treatment in pediatric dentistry. J Calif Dent Assoc 1991; 19: 43–44, 46–48, 50.
Convissar R A, Diamond L B, Fazekas C D . Laser treatment of orthodontically induced gingival hyperplasia. Gen Dent 1996; 44: 47–51.
Sarver D M, Yanosky M . Principles of cosmetic dentistry in orthodontics: part 3. Laser treatments for tooth eruption and soft tissue problems. Am J Orthod Dentofac Orthop 2005; 127: 262–264.
Esen E, Haytac M C, Oz I A, Erdogan O, Karsli E D . Gingival melanin pigmentation and its treatment with the CO2 laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2004; 98: 522–527.
Ishikawa I, Aoki A, Takasaki A A . Potential applications of Erbium:YAG laser in periodontics. J Periodont Res 2004; 39: 275–285.
Tal H, Oegiesser D, Tal M . Gingival depigmentation by erbium:YAG laser: clinical observations and patient responses. J Periodontol 2003; 74: 1660–1667.
Yousuf A, Hossain M, Nakamura Y, Yamada Y, Kinoshita J, Matsumoto K . Removal of gingival melanin pigmentation with the semiconductor diode laser: a case report. J Clin Laser Med Surg 2000; 18: 263–266.
Atsawasuwan P, Greethong K, Nimmanon V . Treatment of gingival hyperpigmentation for esthetic purposes by Nd:YAG laser: report of 4 cases. J Periodontol 2000; 71: 315–321.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Parker, S. Lasers and soft tissue: 'fixed' soft tissue surgery. Br Dent J 202, 247–253 (2007). https://doi.org/10.1038/bdj.2007.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/bdj.2007.172
This article is cited by
-
Mucous membrane healing following vestibuloplasty via 940-nm diode laser and scalpel: an in vivo study
Lasers in Dental Science (2023)
-
Application of 980-nm diode laser for vestibuloplasty: a case series
Lasers in Dental Science (2022)