Abstract
Data sources PubMed, the Cochrane Oral Health Group Trials Register and Embase. Additionally, issues of the following journals between 2000 and April 2019 were hand-searched: Journal of Clinical Periodontology, Journal of Periodontology, International Journal of Periodontology and Restorative Dentistry, European Journal of Oral Implantology, Journal of Oral and Maxillofacial Surgery, Clinical Implant Dentistry and Related Research, and Clinical Oral Implants Research.
Study selection Only randomised controlled trials (RCTs) involving soft tissue augmentation at dental implant sites were considered for inclusion. The selection was restricted to RCTs published in English language with at least ten patients per group and a minimum follow-up period of three months. A PICO method was used to organise the inclusion criteria and soft tissue augmentations were clustered into three groups; that is, before prosthetic treatment, after prosthetic treatment and at immediate implant placement.
Data extraction and synthesis The screening of titles and abstracts was carried out by two reviewers and disagreements were moderated by a third reviewer. Eligibility was determined using full texts and data were extracted using purposefully designed forms. The Cochrane handbook for systematic reviews of interventions toolkit was used to assess the risk of bias. The studies were grouped according to the type of intervention and subjected to quantitative data synthesis. Continuous outcome measures were assessed using random-effects meta-analyses and pooled estimates were expressed as weighted mean differences (MDs) along with 95% confidence intervals (CIs).
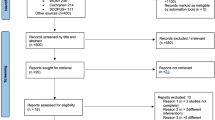
Results Following initial electronic and hand-searches, 2,119 studies were screened for title and abstract, and 32 studies were considered for full-text screening. Only 14 RCTs met the inclusion criteria and the remaining 18 studies were excluded from the systematic review. The included studies described soft tissue augmentation for 538 implants placed in 475 patients. Three studies (68 patients; 78 implants) reported improved soft tissue thickness with xenogenic collagen matrix (XCM) augmentation compared to no augmentation at the implant sites before prosthetic treatment (high/unclear risk of bias). One study (28 patients; 41 implants) reported improved height of keratinised tissue (KT) and marginal bone levels (MBLs) with free gingival graft (FGG) compared to no augmentation at the implant sites after prosthetic treatment (unclear risk of bias). Three RCTs (126 patients; 126 implants) focused on connective tissue grafting (CTG) and bone grafting versus no grafting in conjunction with immediate implant placement after tooth extraction (unclear risk of bias). There was no difference in MBLs in any of the studies, while one study showed superior soft tissue thickness (STT). Four RCTs (129 patients; 133 implants) compared different augmentation techniques before prosthetic treatment. Only one study showed improved STT with CTG compared to XCM (low risk of bias). Finally, three RCTs (124 patients; 160 implants) compared different augmentation techniques after prosthetic treatment (high/unclear risk of bias). FGG was observed to be superior to acellular dermal matrix (ADM) and vestibuloplasty to improve KT. Meta-analyses did not favour CTG to improve MBLs at extraction sites but CTG was superior to XCM to improve STT before prosthetic treatment.
Conclusions Notwithstanding the limitations of the systematic review, soft tissue augmentation significantly enhances the amount of soft tissue at the implant site. CTG at the extraction site also improves subsequent bone level of the implants. Moreover, CTG before prosthetic treatment is superior to XCM to improve thickness of peri-implant soft tissues. However, these findings are based on short-term follow-up and future studies with improved methodology are required to establish the long-term benefits of soft tissue augmentation at the dental implant sites.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lee C T, Huang Y W, Zhu L, Weltman R. Prevalences of peri-implantitis and peri-implant mucositis: systematic review and meta-analysis. J Dent 2017; 62: 1-2.
Papaspyridakos P, Bordin T B, Natto Z S et al. Complications and survival rates of 55 metal-ceramic implant-supported fixed complete-arch prostheses: A cohort study with mean 5-year follow-up. J Prosthet Dent 2019; 122: 441-449.
Berglundh T, Armitage G, Araujo M G et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol 2018; 89: S313-S318.
Schwarz F, Derks J, Monje A, Wang H L. Peri-implantitis. J Clin Periodontol 2018; 45: S246-S266.
Ting M, Craig J, Balkin B E, Suzuki J B. Peri-implantitis: a comprehensive overview of systematic reviews. J Oral Implantol 2018; 44: 225-247.
Jepsen S, Berglundh T, Genco R et al. Primary prevention of peri-implantitis: Managing peri-implant mucositis. J Clin Periodontol 2015; 42: S152-S157.
Ishaque S, Karnon J, Chen G, Nair R, Salter A B. A systematic review of randomised controlled trials evaluating the use of patient-reported outcome measures (PROMs). Qual Life Res 2019; 28: 567-592.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, K., Kay, E. Which type of soft tissue augmentation at dental implant sites is best supported by evidence?. Evid Based Dent 21, 140–141 (2020). https://doi.org/10.1038/s41432-020-0138-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41432-020-0138-y
This article is cited by
-
Does prophylactic application of doxycycline at the implant-abutment interface improve clinical outcomes of dental implants?
Evidence-Based Dentistry (2022)
-
Conventional free-hand, dynamic navigation and static guided implant surgery produce similar short-term patient-reported outcome measures and experiences
Evidence-Based Dentistry (2021)