Abstract
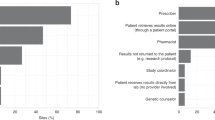
Clinician attitudes toward multiplexed genomic testing may be vital to the success of translational programs. We surveyed clinicians at an academic medical center about their views on a large pharmacogenomics implementation, the PREDICT (Pharmacogenomic Resource for Enhanced Decisions in Care and Treatment) program. Participants were asked about test ordering, major factors influencing use of results, expectations of efficacy and responsibility for applying results to patient care. Virtually all respondents (99%) agreed that pharmacogenomics variants influence patients’ response to drug therapy. The majority (92%) favored immediate, active notification when a clinically significant drug–genome interaction was present. However, clinicians were divided on which providers were responsible for acting on a result when a prescription change was indicated and whether patients should be directly notified of a significant result. We concluded genotype results were valued for tailoring prescriptions, but clinicians do not agree on how to appropriately assign clinical responsibility for actionable results from a multiplexed panel.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
$259.00 per year
only $43.17 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Pulley JM, Denny JC, Peterson JF, Bernard GR, Vnencak-Jones CL, Ramirez AH et al. Operational implementation of prospective genotyping for personalized medicine: the design of the Vanderbilt PREDICT project. Clin Pharmacol Ther 2012; 92: 87–95.
Peterson JF, Bowton E, Field JR, Beller M, Mitchell J, Schildcrout J et al. Electronic health record design and implementation for pharmacogenomics: a local perspective. Genet Med 2013; 15: 833–841.
Shuldiner AR, Relling MV, Peterson JF, Hicks K, Freimuth RR, Sadee W et al. The pharmacogenomics research network translational pharmacogenetics program: overcoming challenges of real-world implementation. Clin Pharmacol Ther 2013; 94: 207–210.
Rasmussen-Torvik LJ, Stallings SC, Gordon AS, Almoguera B, Basford MA, Bielinski SJ et al. Design and anticipated outcomes of the eMERGE-PGx project: a multi-center pilot for pre-emptive pharmacogenomics in electronic health record systems. Clin Pharmacol Ther 2014; 96: 482–489.
Bell GC, Crews KR, Wilkinson MR, Haidar CE, Hicks JK, Baker DK et al. Development and use of active clinical decision support for preemptive pharmacogenomics. J Am Med Inform Assoc 2014; 21: e93–e99.
Bielinski SJ, Olson JE, Pathak J, Weinshilboum RM, Wang L, Lyke KJ et al. Preemptive genotyping for personalized medicine: design of the right drug, right dose, right time-using genomic data to individualize treatment protocol. Mayo Clin Proc 2014; 89: 25–33.
Hoffman JM, Haidar CE, Wilkinson MR, Crews KR, Baker DK, Kornegay NM et al. PG4KDS: a model for the clinical implementation of pre-emptive pharmacogenetics. Am J Med Genet C Semin Med Genet 2014; 166: 45–55.
Van Driest S, Shi Y, Bowton E, Schildcrout J, Peterson J, Pulley J et al. Clinically actionable genotypes among 10,000 patients with preemptive pharmacogenomic testing. Clin Pharmacol Ther 2013; 95: 423–431.
Schildcrout JS, Denny JC, Bowton E, Gregg W, Pulley JM, Basford MA et al. Optimizing drug outcomes through pharmacogenetics: a case for preemptive genotyping. Clin Pharmacol Ther 2012; 92: 235–242.
Stanek EJ, Sanders CL, Taber KAJ, Khalid M, Patel A, Verbrugge RR et al. Adoption of pharmacogenomic testing by US physicians: results of a nationwide survey. Clin Pharmacol Ther 2012; 91: 450–458.
Haga S, Burke W, Ginsburg G, Mills R, Agans R . Primary care physicians’ knowledge of and experience with pharmacogenetic testing. Clin Genet 2012; 82: 388–394.
Haga SB, Carrig MM, O’Daniel JM, Orlando LA, Killeya-Jones LA, Ginsburg GS et al. Genomic risk profiling: attitudes and use in personal and clinical care of primary care physicians who offer risk profiling. J Gen Intern Med 2011; 26: 834–840.
Haga SB, Tindall G, O’Daniel JM . Professional perspectives about pharmacogenetic testing and managing ancillary findings. Genet Test Mol Biomarkers 2012; 16: 21–24.
Hicks JK, Crews KR, Hoffman JM, Kornegay NM, Wilkinson MR, Lorier R et al. A clinician-driven automated system for integration of pharmacogenetic interpretations into an electronic medical record. Clin Pharmacol Ther 2012; 92: 563–566.
Varmus H . Getting ready for gene-based medicine. N Engl J Med 2002; 347: 1526–1527.
Khan NA, Peterson JF . A surveillance tool to support quality assurance and research in personalized medicine. AMIA Annu Symp Proc 2011; 2011: 701–708.
Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG . Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42: 377–381.
Kimmel SE, French B, Kasner SE, Johnson JA, Anderson JL, Gage BF et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N Engl J Med 2013; 369: 2283–2293.
Pirmohamed M, Burnside G, Eriksson N, Jorgensen AL, Toh CH, Nicholson T et al. A randomized trial of genotype-guided dosing of warfarin. N Engl J Med 2013; 369: 2294–2303.
Spurling GK, Mansfield PR, Montgomery BD, Lexchin J, Doust J, Othman N et al. Information from pharmaceutical companies and the quality, quantity, and cost of physicians’ prescribing: a systematic review. PLoS Med 2010; 7: e1000352.
Acknowledgements
We thank Lisa Price for assisting with the survey. This project was funded by Vanderbilt University, the Centers for Disease Control and Prevention (U47CI000824), the National Heart, Lung, and Blood Institute (U01HL122904, U01HL105198), the National Institute for General Medical Sciences (U19HL065962), the National Human Genome Research Institute (U01HG006378), the National Center for Advancing Translational Sciences (UL1TR000445), KL2TR000446 and NICHD K23 HD000001. The analyses described herein are solely the responsibility of the authors alone and do not necessarily represent official views of the Centers for Disease Control and Prevention or the National Institutes of Health. In addition, the funding sources had no role in the study design, the collection, analysis and interpretation of data, manuscript preparation or the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Peterson, J., Field, J., Shi, Y. et al. Attitudes of clinicians following large-scale pharmacogenomics implementation. Pharmacogenomics J 16, 393–398 (2016). https://doi.org/10.1038/tpj.2015.57
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/tpj.2015.57
This article is cited by
-
Combining familial hypercholesterolemia and statin genetic studies as a strategy for the implementation of pharmacogenomics. A multidisciplinary approach
The Pharmacogenomics Journal (2022)
-
Translating pharmacogenomics into clinical decisions: do not let the perfect be the enemy of the good
Human Genomics (2019)
-
Application of pharmacogenetics in clinical practice: problems and solutions
Journal of Neural Transmission (2019)
-
On the readiness of physicians for pharmacogenomics testing: an empirical assessment
The Pharmacogenomics Journal (2018)
-
Challenges and strategies for implementing genomic services in diverse settings: experiences from the Implementing GeNomics In pracTicE (IGNITE) network
BMC Medical Genomics (2017)