Key Points
-
On many occasions the maxillary sinus (antrum) is in close proximity to surgical sites in the mouth and can therefore become involved in operative complications.
-
The clinician must be familiar with common pathology affecting the maxillary sinus.
-
An outline of the pathological conditions affecting the maxillary sinus is provided.
-
Indications for sinus surgery are outlined.
Abstract
The maxillary sinus is the largest of the four paranasal sinuses and, being anatomically adjacent to the dentate region of the maxilla, is commonly a source of problems – not simply in terms of conditions affecting the sinus but also in establishing an accurate diagnosis. As anyone who has suffered both sinusitis and a dental abscess in the posterior maxilla will tell you, the symptoms are almost indistinguishable. For this reason, a sound understanding of the maxillary sinus is an essential requisite for all dentists.
Similar content being viewed by others
Anatomy
The human skull has four pairs of sinuses. These are air-filled cavities adjacent to the nasal region and collectively may be referred to as the paranasal sinuses.
-
Frontal sinus
-
Sphenoidal sinus
-
Ethmoidal sinus
-
Maxillary sinus (also referred to as the antrum of Highmore or maxillary antrum).
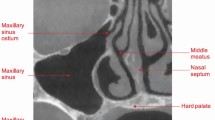
Figure 1 illustrates the anatomical position of the sinuses. The maxillary sinus is a large pyramidal chamber within the maxillary bone and is lined with respiratory (pseudo-stratified ciliated) epithelium (also known as the Schneiderian membrane). Its function is uncertain but it has been suggested that it moistens inspired air, lightens the skull and possibly provides vocal resonance. The terms maxillary sinus and antrum of Highmore are frequently abbreviated simply to sinus and antrum and are used synonymously, although maxillary sinus is the preferred terminology in anatomical circles.
All paranasal sinuses slowly pneumatise from birth to adulthood.
The maxillary sinus is pyramidal in shape, with its base on the palate, three sides (lateral nasal wall, posterior antral wall and anterior maxillary wall), and its apex laterally in the zygomatic process (Fig. 2). The roof of the sinus forms part of the floor of the orbit and is therefore related to the infraorbital vessels and nerves. The floor/base of the sinus can project between the roots of the maxillary premolar and molar teeth (Fig. 3). Medially, the sinus is separated from the nasal cavity by a thin wall of bone/cartilage, and it drains through ciliary action into the nose high on the lateral wall through an ostium in the middle meatus (Fig. 2). The height of this ostium means that (in upright posture) sinus drainage is poor in the presence of inflammation or after surgical intervention; for example, sinus grafting. Traditionally, increasing the size of this ostium was a routine part of ear, nose and throat (ENT) surgery to improve drainage from the maxillary sinus. However, this has a detrimental effect on the ciliary motion, thus compromising natural sinus drainage. The development of minimal access surgery (functional endoscopic nasal surgery) largely avoids the need for invasive surgery.
In the presence of infection, blockage of the lacrimal duct and occlusion of the maxillary antral space may cause epiphora (excessive tear formation), decreased ocular movement and/or exophthalmos. The proximity of the infraorbital nerve and superior alveolar nerves results in pain, which can be quite severe. However, if there is direct nerve injury due to trauma or neoplasia there may be anaesthesia, altered sensation and/or pain. In itself, pain in the region of the maxillary sinus may be due to a number of causes including: sinusitis, tumour, myofascial pain, trigeminal neuralgia, atypical facial pain or periodic migrainous neuralgia.
Increasing pneumatisation with age concomitant with any alveolar ridge resorption due to loss of teeth may result in there being little bone between the dental alveolus and the floor/base of the maxillary antrum.1
The maxillary antrum: surgery
Surgery involving the maxillary antrum may be indicated for implant surgery, operative complications or pathology. This article (book chapter) will concentrate solely on the latter indications as implant surgery is covered in a book in the British Dental Association's clinical guide series: A clinical guide to implants in dentistry.2
The most common operative complications involving the antrum are:
-
Creation of an oroantral communication
-
Fractures of the maxillary alveolus or tuberosity
-
Extraneous objects (tooth, root, implant or foreign body) displaced into the maxillary antrum.
Oroantral communication and oroantral fistula
Definitions:
Oroantral communication (OAC): An open communication between the oral cavity and the maxillary sinus.
Oroantral fistula (OAF): Communication between the oral cavity and the maxillary sinus lined by epithelium.
Several studies have highlighted the proximity of the maxillary tooth roots to the floor of the maxillary sinus. In 1925 Von Bondsdorff3 studied 84 human skulls and found that second molar roots have the most intimate relationship with the floor of the maxillary antrum, followed by the first molar, third molar, second premolar, first premolar and the canine.
The factors of importance are:
-
The antral floor in this region is thin: 1–7mm.4
-
Bone resorption is related to chronic apical periodontitis.5
-
Early root development and complete root development also increase the risk of OAC.5
It has been suggested that many maxillary posterior teeth extractions result in an immediate micro-communication between the mouth and the maxillary antrum, but that most of these OACs are subclinical and therefore remain undetected by patient and clinician, healing spontaneously of their own accord without operative intervention.1
Predisposing factors for an OAC
Several factors can predispose a patient to an OAC during dental extraction:
-
The proximity of the relationship of the antrum to the teeth (OAC can occur from the premolar to the molar region, although the latter is more common)
-
Infra-occluded (submerged) teeth
-
Lone-standing molar tooth
-
Hypercementosis, tooth shape: bulbous roots or bony sclerosis
-
Loss of periapical bone due to periodontal disease, periapical granuloma or a cyst
-
Surgery performed within or in close proximity to the maxillary sinus
-
Impacted maxillary third molars (greater surgical difficulty and proximity to sinus floor)
-
Intraoperative root fractures
-
Fracture of maxillary tuberosity (more likely with extraction of isolated posterior teeth and third molars)
-
Use of excessive force.5
An oroantral fistula (OAF) is the result of an OAC that has failed to heal spontaneously, and the connection between the mouth and sinus cavities has epithelialised to form a fistula connecting the two spaces (Fig. 4a). The presence of a dental abscess, cyst or foreign body may all prevent spontaneous closure of the OAC5 and it is also less likely when the defect is greater than 5 mm.6
a) Extracted upper first molar with divergent roots and local alveolar bone. b) Identified OAC via the socket of the upper right extracted first maxillary molar. c) Antral lining apparent via the upper right first molar socket at extraction. d) Direct buccal mucosal closure of OAC. e) Using a scalpel to incise the buccal flap periosteum to advance the flap over the socket to attain passive closure. f) Buccal mucosal advancement flap closure of OAC identified at extraction
Chronic oroantral fistulae may present as obvious defects or as antral polyps, consisting of maxillary antral lining herniating through the communication and becoming visible in the mouth (Fig. 4b). Patients with cleft lip and palate are more prone to OAFs because of the congenital defects and the associated significant soft tissue deficiencies that prevent simple closure of the defects (Fig. 4c).
Diagnosis of OAC at the time of surgery
If the patient breathes in and out through the nose, the antral lining at the apex of the socket may be seen to move in time with respiration. Alternatively, if there is a tear in the antral lining, bubbles may be seen emanating from the socket. There may also be a characteristic hollow sound when using aspiration in the socket.
Examination of the extracted tooth may reveal a piece of concave bone or fractured tuberosity attached to the roots. Postoperative radiographs may show a defect in the antral floor, but radiographs are not mandatory to make an acute diagnosis, which can be made mostly on clinical grounds.
Diagnosis of OAF post-surgery
The development of maxillary sinusitis should alert the clinician to the possibility of oroantral fistulas, as odontogenic factors cause 56–70% cases of maxillary sinusitis.7 With liquid able to pass freely between the oral and nasal cavities, the development of infection and suppuration will result in a foul taste/discharge.
An OAF presents with similar signs and symptoms to an OAC, but tends to persist and may be accompanied by:
-
Regurgitation of fluids/food into the nose
-
Nose bleeds (epistaxis)
-
Chronic sinusitis and pain
-
Prolapse of the antral mucosa into the mouth (Fig. 4c).
Radiographs may again be helpful but are not mandatory to make the diagnosis. Conventionally, occipitomental views were used to confirm the presence of sinusitis associated with persistent infection due to an OAF, indicated by unilateral opacity of the maxillary sinus (Fig. 5d). Most ENT surgeons prefer the use of coronal CT views to confirm the patency of the ostium (Fig. 5b) but cone beam CT (CBCT) can also be used (Fig. 5e). The identification and location of any displaced root is also much more accurate with the use of CT or CBCT, and so these are now the preferred options.
b) Coronal CT section through the orofacial region illustrating an opaque left maxillary sinus indicating mucosal thickening or a lesion. c) Axial CT section through the lower maxilla showing left maxillary antral sinus congestion with mucosal thickening. d) Occipitomental view (20 degrees) illustrating bilateral clear maxillary antrum. e) CBCT showing a large cystic lesion of the left maxillary sinus presenting as opacity on DPT
Incidence
OACs are most common during the extraction of upper first and second molars, especially if lone-standing, and are most common in the 3rd and 4th decades.8 Small OACs usually resolve spontaneously, provided they are given the chance to heal. In this regard, most clinicians advise patients to refrain from blowing their nose for a period of 2-3 weeks and recommend excellent oral hygiene.
Management of OACs and OAFs
There are many varied recommendations for the management of OACs and OAFs.9
Conservative treatment
Options for an OAC include:
-
Gentle irrigation of the socket, debridement of any sharp bone and removal of any local irritant factors (e.g. plaque/calculus on adjacent teeth)
-
Suturing any loose edges of the wound so that the mucoperiosteum is in close apposition to the bone
-
Consideration to placing a resorbable haemostatic agent such as Surgicel (oxidised regenerated cellulose) into the socket to help with clot formation and to provide a matrix for maintenance of the clot during healing. The disadvantages of this are:
-
Such a material will be identified as foreign and may provoke a foreign body reaction in a minority of patients
-
It has a low pH and can cause postoperative pain.
-
Consideration to taking an alginate impression of the area with protective gauze over the OAC for the manufacture of an upper vacuum-formed splint to protect the area. This can then be worn 24 hours a day for 2 weeks (removal only for cleaning to provide protection to the affected area while early healing occurs)
-
The possibility of prescribing an 'antral regimen' and careful review. Avoiding an OAC persisting and becoming an OAF will involve minimising maxillary sinusitis post-extraction to allow an OAC to heal. Antral regimens are controversial and include the possible use of antibiotics and nasal decongestants as adjuncts to good oral hygiene and the prescription of chlorhexidine mouthwash.
If a fistula appears to be developing despite conservative management it may be necessary to intervene surgically.
Surgical management options for the closure of an established OAF
A literature review10 highlights the factors that influence the success of the surgical management of OAFs, which include:
-
Size of defect
-
Previous surgery
-
Medical status of the patient
-
Smoking status of the patient
-
Oral hygiene
-
Pre-existing sinus pathology
-
Experience of the operator
-
Surgical technique used.
All of these factors must be considered and where possible rectified. The prior treatment of sinusitis due to the OAF must be addressed preoperatively. Guidelines for the management of sinusitis include prescribing antibiotics,11 oral hygiene and decongestants.
Whichever surgical management is used to close an OAF there is usually a requirement for some sort of 'antral regimen' pre- and postoperatively and consideration should also be given to whether or not the repair performed requires any protection through the use of a vacuum-formed soft splint or a hard acrylic cover plate.
The principles for the surgical management of OAFs consist of:
-
Excision of the epithelially lined fistula
-
Repair of the resultant defect.
Visscher et al.10 have reviewed the possible surgical strategies for closing OAFs (Table 1).
First, excision of the fistula is required and frequently reveals a much larger bony aperture than was clinically apparent. Using a dental probe the bone margins can be mapped out prior to incision. The most appropriate closure techniques should be used to help repair the resultant defect. Of the various techniques available, the two most commonly used are the buccal advancement flap and buccal fat pad advancement.
Buccal advancement flap
This is the accepted first choice method for the repair of OACs and OAFs:
-
It is the commonest technique, first described by Rehrmann in 193612
-
It involves excising the fistula and lifting a broad-based trapezoid flap, and incising the periosteum horizontally to allow tension-free closure. The flap is sutured to the palatal mucosa using mattress sutures
-
There is a high success rate of 93%13
-
Disadvantages include decreased buccal sulcus depth, problems with future prosthetic rehabilitation, and postoperative pain and swelling.6
Technique: After excision of the fistula, a three-sided buccal mucoperiosteal flap is lifted in the area immediately adjacent to the defect. The periosteum on the underside of the flap is then carefully incised horizontally near the base of the flap. This allows the periosteum to part as the mucosa is advanced over the defect and sutured without tension to the palatal side (Fig. 6). In larger defects, a series of horizontal cuts through the periosteum may be made, thus allowing a greater 'concertina' effect to advance the flap over the opening. The essential factors in success of this treatment are complete coverage of the defect with a tension-free flap and eversion of the flap margins with the judicious use of horizontal or vertical mattress sutures.
a) Oroantral defect of upper left 6 socket; mesial and distal incisions including the socket defect indicated. b) Axial view of the defect and the elevated buccal advancement flap. c) Raised flap near-vertical (with a slight angle for a wider base and to optimise the blood supply. Ensure the oroantral fistula track is carefully excised to ensure fresh clean margins for repair. d) Axial view. e) Once the buccal flap is elevated, underscore the periosteum using a blade lightly to advance the buccal flap over the socket. The aim is to get the mucosal flap passively sitting over the fistula to maximise healing without tissue tension. It is not generally recommended to perform an 'alveolectomy' to improve passive advancement of the mucosal flap, as the authors would promote bone retention where possible, but this is an option. f) Axial section of the buccal advancement flap sutured passively in place
Buccal fat pad advancement
The buccal fat pad is a vascularised, lobulated mass of fatty tissue surrounded by a slight capsule, located in the maxillary molar region. The body of fat is positioned in the buccal tissue space (Fig. 7), with its bulk lying below and anterior to the zygomatic arch and slightly superior to and more distal than the occlusal level of the second and third maxillary molar teeth.
Some features of the buccal fat pad include:
-
Its size is relatively constant, regardless of fat distribution and body weight14
-
Blood supply to the pad is via branches of the superficial temporal, maxillary and facial arteries
-
It is accessed via vestibular incision and blunt dissection. The pad is then transposed into the defect and sutured to the margins. The fat pad is sutured back into original position (no periosteal relief used).
The buccal fat pad flap was first described by Egyedi in 1977.15
Technique: It can be approached using a buccal mucoperiosteal flap and horizontal periosteal relief followed by careful blunt dissection into the buccal space. Once identified it frequently herniates through the periosteum, although further blunt dissection may be necessary to free it enough to allow suturing into position over the defect created by excising the OAF (Fig. 8). It can then either be left to epithelialise or a two-layer closure can be completed by advancing the buccal mucoperiosteal flap over it and suturing this to the palatal mucosa.
a) Buccal incision, starting from the defect superiorly and posteriorly up into the buccal sulcus. b) Continue the incision down through the periosteum of the attached gingivae and up into approximately 1 cm of non-attached gingivae. c) Elevate subperiosteally under the mesial incision, reflecting the flap away from the buccal aspect of the lateral maxillary wall. d) Incise into the periosteum high under the zygomatic arch to release the buccal fat pad. Incise approximately 1 cm with a blade initially, then use curved McIndoe's scissors or an artery clip to carefully perform a blunt dissection superiorly and laterally until the fat pad herniates through the periosteum. e) Once the fat pad is exposed and released, continue careful blunt dissection to elevate it without compromising the stem (whereby the base or vascular supply will be lost). f) Continue dissection until the fat pad sits passively over the oroantral defect. g) Suture the fat pad passively over the defect, and ideally close it with an adjunctive second layer (h) mucosal advanced flap). i) and j) axial views
Advantages of the buccal fat pad flap include:
-
Close to surgical site, quick procedure
-
Good blood supply
-
Buccal sulcus depth unaffected, high success rate.
Disadvantages include the possibility of:
-
Increased postoperative pain and swelling
-
Reduction in mouth opening
-
Partial necrosis.
Comparison of the pedicled buccal fat pad flap with the buccal flap for the closure of OACs found high success rates for both techniques.16
The next two techniques are far less commonly used but can be useful when the previous techniques discussed have proved unsuccessful or are inappropriate.
Palatal pedicle rotation flap (or palatal finger flap)
-
Indicated in larger defects or where there has been previous failure
-
First described by Ashley in 193917
-
Utilises a flap based on the greater palatine artery, which has a good blood supply and thick mucosa
-
A broad-based partial thickness flap that extends anterior to the defect is rotated to cover the defect
-
Leaves a denuded area in the palate covered by periosteum
-
Postoperative pain, discomfort and necrosis can occur if there is excessive rotation
-
The patient will be unable to wear an upper denture for quite some time.18
Technique: Following excision of the fistula, a mucoperiosteal flap is raised based on the greater palatine artery. It can then be carefully rotated to close defects in the second premolar and first molar regions. Repairs attempted with this flap any further anterior or posterior may compromise the blood supply to the flap and therefore may fail. The bare area of palate will re-epithelialise spontaneously, although it can be covered with a dressing such as Whitehead's varnish on a gauze pack or a proprietary dressing such as Coe-Pak. Sufficient space should be left below any dressing to allow a coagulum to form and healing by secondary intention to occur (Fig. 9). This technique is no longer commonly used but is still useful if the first two methods prove unsuccessful.
Tongue flap
This involves the raising of a finger flap from the tongue and, while it is still attached to the tongue, suturing the tip of the flap into the defect left by the excision of the fistula. The flap is left like this, usually with the patient in intermaxillary fixation for 2-3 weeks, at which point it is divided from its base and the remainder of the flap is sutured into place and the defect on the tongue closed primarily. Needless to say, tongue flaps are unpleasant for the patient and used only in very limited circumstances, often being reserved for extremely large defects that cannot be managed in other ways.
Emerging surgical management techniques for the closure of an established OAF
Autogenous bone grafting
This approach may be useful if future implant treatment is planned. Bone grafts can be harvested from the retromolar area for 'press fit' closure of an OAC19 or alternatively a chin graft can be used.20 However, this requires two-site surgery resulting in increased postoperative pain and discomfort
Palatal connective tissue flap
This technique may be used as an adjunctive layer when closing an OAF but is not yet common practice.
Fractures of the maxillary alveolus or tuberosity
Bell in a paper dedicated to the management of fractured tuberosity, highlights in detail issues in prevention and management.21 The fractured tuberosity is a common complication, particularly in the following situations:
-
Extractions in older patients, especially of lone-standing upper molar teeth
-
Removal of (impacted) maxillary third molar teeth
-
Removal of maxillary molar teeth with very divergent roots or with hypercementosis
-
Removal of maxillary posterior teeth in the presence of a very pneumatised maxillary antrum.
The use of excessive force while extracting teeth in the situations listed above should be avoided. When undertaking an extraction, if the tooth and tuberosity or maxillary alveolus are felt to move synchronously with the movement of the extraction technique applied (often accompanied by a large tear in the palatal mucosa and/or fracturing noise), a decision must then be made on management, which broadly falls into three possibilities:
-
Rigidly splint the tooth in situ and return to the extraction using a surgical technique in 6-8 weeks. This approach to managing the fracture may be the preferred option when the fracture is very large and incorporates either the antral floor or adjacent sound teeth. When using this approach consideration will have to be given to palliating the tooth being extracted, i.e. extirpation or antibiotics as necessary
-
Removal of the fractured tuberosity. This can be accomplished by careful subperiosteal dissection of the mucoperiosteum off the fragment. If the fragment is very large and involves adjacent teeth it may be that a surgical approach will be required, gaining access with a mucoperiosteal flap that minimises the amount of periosteal stripping over the segment to be retained. The piece to be removed can then be surgically divided from the piece to be retained (Fig. 10)
-
Proceed with the extraction by surgically separating the tooth from as much of the bone as possible. This technique is difficult and is dependent on there being an adequate piece of residual bone with an undisturbed blood supply. If this technique fails, it usually results in a huge defect that can be difficult to close and which results in a significant loss of bone from the posterior maxilla, making the subsequent construction of upper dentures in the future extremely difficult.
In all cases the patient must be informed of the intraoperative complication immediately post-surgically and be followed up accordingly.
Extraneous objects in the sinus
The displacement of tooth fragments, root canal medicaments or surgical instruments into the antrum is usually avoidable, especially if correct extraction techniques are applied. Avoiding the blind placement of forceps and elevators is imperative, and seeking to grasp small pieces of upper roots with forceps can cause an 'orange pip' effect in which the root is forced upwards from the inadequate grip of the forceps beaks. Roots tend to be more commonly displaced than teeth, especially the palatal roots of maxillary molars (Fig. 11a). Fractured roots may also be displaced into the space between the epithelial lining and the antral bone. It is therefore important to establish a correct diagnosis when displacement is suspected. There are several reports of displacement of implants into the maxillary antrum (Fig. 11b), some of which have resulted in litigation.
Immediate removal of these foreign bodies is recommended when possible. If a tooth fragment cannot be located, even if there is an obvious OAC present, care should be taken to ensure that it is not lying just under the mucosa (palatally or buccally). The lingual sulci and oropharynx should also be examined, as should the suction apparatus if it is being used. It is also important to check that the patient did not feel themselves swallow or aspirate the root fragment as, although one would assume this would be obvious to the operator at the time of the surgery, this is not always the case.
The position of the root should then be identified by the appropriate use of radiographs. Periapical, occipitomental or occlusal views can all be useful in ensuring that the root is in the sinus and either free or trapped under the antral lining. As mentioned earlier, the best means of localising a root in the sinus is by using CT or CBCT. If a root cannot be located in the oral cavity, oropharynx, suction or sinus, then consideration should be given to performing a chest X-ray to ensure that it has not been aspirated, especially if the patient's cough reflex could be depressed for any reason.
Management of a displaced root/tooth
If there is an OAC in the socket of the root in question it is reasonable to gently attempt removal through the defect with a small fine-bore suction tip. There is no point in persisting with this for any length of time and consideration should be given to raising a mucoperiosteal flap to improve vision and access. The more astute practitioner may prefer to refer the patient to a specialist at this stage rather than prolong the patient's discomfort.
Dependent on the position and fixed nature of the root at the time of displacement, specialist oral surgeons may choose to enlarge the existing OAC and approach the removal of the root through this. The advantage of this approach is that it uses the existing OAC and does not create another defect in the antral wall; its disadvantage is the poor and limited access to the antrum it affords in comparison to an anterior approach such as the Caldwell–Luc approach (Fig. 12). After the root is removed the OAC can then be closed using any of the options listed for the management of an OAF.
If a formal surgical approach is required for the removal of a tooth from the sinus then the Caldwell–Luc approach or functional endoscopic sinus surgery (FESS) may be indicated. General indications for the Caldwell–Luc approach include the removal of both foreign bodies and odontogenic tumours or cysts (from tooth-forming tissues). The procedure involves raising a buccal flap (either crescent in the non-attached gingivae or including attached gingivae; Fig. 12b) then, after exposing the anterior maxillary wall, creating a bony window above and slightly posterior to the apex of the canine tooth. The antral lining is often perforated simultaneously, thus providing adequate access for the removal of lesions or foreign bodies. The window allows good direct vision of the sinus from a frontal position. Routine closure of the flap with interrupted sutures is recommended, with provision of an antral regimen and advice to the patient not to blow their nose for 3 weeks.
Endoscopic sinus surgery has also become a useful technique for accessing the maxillary sinus. Via an enlarged middle meatal antrostomy, the whole of the maxillary sinus can be accessed, except for lesions involving the anterior wall.
The maxillary sinus: pathology
The types of pathological conditions that can affect the maxillary antrum include:
-
Infection (sinusitis)
-
Mucous retention cysts
-
Odontogenic and non-odontogenic cysts
-
Genetic, metabolic and tumour-like diseases
-
Benign or malignant neoplasms.
A summary of the various pathologies that may present in the maxillary sinus has been published.8
Maxillary sinusitis
Inflammation of the maxillary sinus (sinusitis) can occur as an acute condition or in a chronic form. The two main causes of acute maxillary sinusitis are decreased drainage, usually due to obstruction of the ostium, and/or overwhelming infection. This may be viral, as a result of a common cold or viral influenza, or bacterial, secondary to severe apical infection or bacterial nasal infection. Fungal infections are probably rare other than in immunocompromised individuals.
Predisposing factors to sinusitis include a deviated nasal septum, an immunocompromised or debilitated patient and the degree of virulence of the infection. Previously, occipito-mental views were used to identify severe persistent maxillary sinusitis (see Fig. 11a). Mucosal thickening can frequently be identified on routine panoral radiographs but CT (Fig. 13) is often used by ENT surgeons for evaluation of the maxillary sinus as it is believed to exaggerate sinus inflammatory disease less than MRI.
Acute sinusitis is characterised by a moderate to severe constant pain over the antrum/cheek area, which may be mistaken for dental pain. Other features include pyrexia, tenderness, especially when the head is moved (going down stairs or leaning forward), mucopurulent discharge from the nose, facial swelling and oedema of the cheek and teeth, which are tender to percussion if they are adjacent to the sinus. Unless the tooth is the primary cause of the sinusitis, however, electric pulp testing usually reveals a positive (vital) response.
Treatment, when required, normally consists of a broad-spectrum antibiotic, nasal decongestants, inhalations and analgesics. Subsequent surgical treatment may include antral washouts for sinuses unresponsive to medical treatment and/or FESS. However, in cases where the sinusitis is secondary to a viral infection, it will usually resolve spontaneously and only analgesics are required.
If not treated promptly, acute sinus infection may spread to the other paranasal sinuses or even result in laryngitis, otitis media (infective organisms traversing the Eustachian tube) or pneumonia. A more common risk is that chronic sinusitis develops.
Chronic sinusitis is relatively uncommon and indicates a persisting inflammation of the sinus, usually for at least 3 months. Predisposing factors, other than an episode of acute infection, include poor drainage, nasal polyps, deviated septum due to trauma/cocaine abuse/virulent infection, smoking, decreased resistance, asthma and allergic rhinitis.
Chronic sinusitis is characterised by blockage of the nasal passage(s) and a runny nose, which may have a mucopurulent discharge. A reduced sense of both smell and taste is often reported and a dull, nagging pain over the sinuses persists, although it is considerably less severe than that of acute sinusitis. Dental pain and headaches may also be present. Other features can include halitosis, swelling of the cheek, increased somnolence (snoring) and earache.
Management
Management of chronic sinusitis is not always straightforward. Attempts to surgically cleanse the sinuses are rarely successful and should not be attempted. However, the use of FESS to improve antral drainage and to remove any obstruction adjacent to the ostium has shown to be an effective treatment with 90% of patients responding when medical treatment has failed. Dilation of the ostium can also be undertaken using a balloon catheter and this also appears to be a potentially useful technique, although further research is indicated as to its true effectiveness.
Long-term antibiotics11 (up to 6 weeks of doxycycline or a macrolide) and topical nasal steroids are the most successful forms of medical treatment for chronic sinusitis. The use of topical nasal decongestants for the treatment of chronic sinusitis should be discouraged as there tends to be a rebound effect that actually makes matters worse. Fungal infections of the maxillary sinus are rare but do occasionally cause chronic sinusitis; treatment consists of a medium-term course of an antifungal medication such as miconazole or fluconazole. FESS and topical steroids should be used if the infection is localised (and usually secondary to allergic rhinitis) and the individual is otherwise healthy.
Complications of chronic sinusitis are rare, although the condition is unpleasant to live with. The risk of infection spreading is the main hazard. Acute or chronic maxillary sinusitis may spread to other sinuses. Dental pathology should always be excluded as a possible cause of maxillary sinusitis.
Maxillary sinus cysts
There is considerable variation in what could be termed the normal radiography of the maxillary sinus.22 In addition, radiographic changes are common and may include mucosal thickenings (in around a third of cases) and maxillary sinus cysts (MSCs; in 8.5% of cases) (Fig. 14). The differential diagnosis of MSCs includes pseudocysts, mucous retention cysts and mucocoeles.8 Interestingly, a relationship between common MSCs and thickening of the antral lining in dentate patients has been confirmed.9
Once the diagnosis has been made, these lesions largely follow an asymptomatic and benign course and usually require no surgical intervention.
Cysts: odontogenic and non-odontogenic
A number of cysts of differing origin (inflammatory and odontogenic) occur that can affect the maxillary sinus. Two examples are given that show the variation in presentation and the potential difficulty in differential diagnosis:
Radicular cyst originating from the carious root of maxillary teeth: Figure 15 shows a clearly visible well-delineated unilocular homogeneous radiolucency. The cyst has grown so large that it has caused displacement of the ipsilateral anterior wall and floor of the maxillary sinus. Although displacing the sinus wall, it is not technically a sinus cyst, however.
Keratocystic odontogenic tumour (KCOT): This relatively rare tumour is accompanied by painless expansion of the maxilla, complicated by sinusitis. Sporadic (non-syndromic) and syndromic KCOTs are associated with mutations in the PTCH gene, which is part of the 'hedgehog' signalling pathway.5 Treatment for keratocysts may involve several different approaches depending on the location, size and multiplicity of the lesion(s) (see Chapter 3 of the BDJ book: A clinical guide to oral surgery – Book 2.
Genetic, metabolic and tumour-like diseases affecting the maxillary antrum
Several of these conditions may involve the maxillary sinus, including congenital clefts, fibrous dysplasia, Paget's disease and osteoporosis. Two examples are given below:
Fibrous dysplasia: The lesion is radio-opaque when in the sinus (compared with the 'orange peel' appearance that occurs within bone) and has some radiolucent mottling. It is often described as having a ground (or frosted) glass appearance. The lesion often melds with the normal surrounding bone. If there are multiple bones involved (polyostotic disease) consider McCune–Albright syndrome (café au lait spots/endocrine disorders/polycystic ovaries, precocious puberty and pituitary adenoma).
Paget's disease of bone (PDB): is the second most common metabolic bone disease following osteoporosis. It is most common after the age of 60 years, with a prevalence of around 3% in this age group. PDB is characterised by an increase in bone resorption and bone deposition, resulting in bone pain and deformities. For this reason, antiresorption drugs that reduce the depletion of calcium and other minerals were originally indicated as the ideal therapy for PDB. The availability of newer, more potent nitrogen-containing bisphosphonates has improved treatment outcomes, allowing the more convenient management of this disorder. However, no firm evidence exists to show that bisphosphonates can prevent the complications of PDB. Patients with PDB may still complain of deafness or other cranial nerve neuropathies associated with sclerosis of skull foramina if these areas are involved in the disease process. Obliteration of the cranial sinuses and enlargement of dental ridges are common. The radiographic features of craniofacial PDB include cotton-ball radio-opaque sclerotic deposits, radiolucent areas and loss of periodontal lamina dura. The key clinical significance of this condition is the use of bisphosphonates and the significant risk of haemorrhage due to increased vascularity.
Neoplasia: benign and malignant
There are many different neoplastic lesions that can affect the maxillary sinus.
Carcinoma: Figure 16 shows a carcinoma of left maxillary antrum, discovered in a patient who was complaining of mobile and painful maxillary teeth/roots. Other features of any fast-growing, space-occupying lesion of the maxillary antrum may include orbital floor displacement, diplopia, nasal obstruction or discharge, palatal swelling, cheek numbness and/or pain. Radiographically, the irregular loss of defined borders and outline should always give cause for concern. Sinister symptoms, particularly spontaneous neuropathy, must not be overlooked and urgent referral (maximum 2-week wait) should be secured.23
Summary
Sinus surgery is occasionally indicated for OAC/OAF and the retrieval of displaced roots. The clinician needs to be familiar with normal anatomy (including acceptable variation) as well as knowing the common conditions that may affect the sinus. This should prevent misdiagnosis and poor management of patients. Over the last decade, significant advances have been made in sinus surgery and the once-common practice of direct access by large antrostomy of the anterior maxillary wall is avoided by FESS. It is now recognised that when the sinus and nasal walls are significantly disrupted, the function of the ciliary and respiratory epithelium is severely compromised. The other aspect of sinus surgery – in relation to implants – is covered in the British Dental Association's book A clinical guide to implants in dentistry.2
References
Mathew A L, Pai K M, Sholapurkar A A . Maxillary sinus findings in the elderly: a panoramic radiographic study. J Contemp Dent Pract 2009; 10: E041–48.
Palmer R, Howe L, Palmer P . A clinical guide to implants in dentistry. London: British Dental Association, 2008.
Von Bondsdorff P . Untersuchungen uber Massverhaltnisse des Oberkiefers mit spezieller Berucksichtigung der Lagebeziehungen zwischen den Zahnwurzeln und der Kieferhohle. Thesis, Helsinki, 1925. Cited in Punwutikorn J, Wailkakul A, Pairuchvej V. Clinically significant oroantral communications: a study of incidence and site. Int J Oral Maxillofac Surg 1994: 23: 19–21.
Skoglund L A, Pedersen S, Hoist E . Surgical management of 85 perforations to the maxillary sinus. Int J Oral Surg 1983: 12: 1–5.
Rothamel D, Wahl G, d'Hoedt B, Nentwig GH, Schwarz F, Becker J . Incidence and predictive factors for perforation of the maxillary antrum in operations to remove upper wisdom teeth: prospective multicentre study. Br J Oral Maxillofac Surg 2007; 45: 387–391.
von Wowern N . Correlation between the development of an oroantral fistula and the size of the corresponding bony defect. J Oral Surg 1973; 31: 98.
Geiger S A, Eckert H . Klinische und radiologische Untersuchungsergebnisse operierter Kieferhöhlen (Clinical and radiological findings in the surgically treated maxillary sinus). Zahnarztl Prax 1975; 26: 296–300.
Bell G W, Joshi B B, Macleod R I . Maxillary sinus disease: diagnosis and treatment. Br Dent J 2011; 210: 113–118.
Abuabara A, Cortez A L, Passeri L A, de Moraes M, Moreira R W . Evaluation of different treatments for oroantral/oronasal communications: experience of 112 cases. Int J Oral Maxillofac Surg 2006; 35: 155–158.
Visscher S H, van Minnen B, Bos R R M . Closure of oroantral communications. A review of the literature. J Oral Maxillofac Surg 2010; 68: 1384–1391.
Drug prescribing for dentistry: dental clinical guidance. Dundee: Scottish Dental Clinical Effectiveness Programme, 2008. http://www.wales.nhs.uk/sites3/Documents/781/1000%20Lives%20PB%2002%2002B%20-%20SDCEP%20Drug%20Prescribing%20for%20Dentistry.pdf.
Rehrmann V A . Eine methode zur schliessung von kieferhohlen perforationen. Dtsch Zahnarztl Z 1936; 39: 1136–9. Cited in Sokler K, Vuksan V, Lauc T. Treatment of oroanthral fistula. Acta Stomatol Croat 2002: 36: 135–140.
Killey H C, Kay L W . Observations based on the surgical closure of 362 oro-antral fistulas. Int Surg 1972; 57: 545.
Stuzin J M, Wagstrom L, Kawamoto H K, Baker T J, Wolfe S A . The anatomy and clinical applications of the buccal fat pad. Plast Reconstr Surg 1990; 85: 29–37.
Egyedi P . Utilization of the buccal fat pad for closure of oro-antral and/or oro-nasal communication. J Maxillofac Surg 1977; 5: 241–4.
Nezafati S, Vafaii A, Ghojazadeh M . Comparison of pedicled buccal fat pad flap with buccal flap for closure of oro-antral communication. Int J Oral Maxillofac Surg 2012; 41: 624–8.
Ashley 1939, as cited by Amaratunga N A. Oro-antral fistulae – a study of clinical, radiological and treatment aspects. Br J Oral Maxillofac Surg. 1986; 24: 433–437.
Borgonovo A E, Berardinelli F V, Favale M, Maiorana C . Surgical options in oroantral fistula treatment. Open Dent J 2012; 6: 94–98.
Watzak G, Tepper G, Zechner W, Monov G, Busenlechner D, Watzek G . Bony press-fit closure of oro-antral fistulas: a technique for pre-sinus lift repair and secondary closure. J Oral Maxillofac Surg 2005; 63: 1288–94.
Haas R, Watzak G, Baron M, Tepper G, Mailath G, Watzek G . A preliminary study of monocortical bone grafts for oroantral fistula closure. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2003; 96: 263–266.
Bell G . Oro-antral fistulae and fractured tuberosities. Br Dent J 2011; 211: 119–123.
Vallo J, Suominen-Taipale L, Huumonen S, Soikkonen K, Norblad A . Prevalence of mucosal abnormalities of the maxillary sinus and their relationship to dental disease in panoramic radiography: results from the Health 2000 Health Examination Survey. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2010; 109: e80–87.
Parmar S, Chapple I L . Late diagnosis of an occult tumour: what lessons can we learn? Br Dent J 2012; 212: 531–534.
Acknowledgements
Our thanks to Mr David Roberts, ENT Consultant Kings Health Partners for reviewing the chapter.
Author information
Authors and Affiliations
Corresponding author
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Renton, T., Durham, J. & Hill, C. Oral surgery II: Part 2. The maxillary sinus (antrum) and oral surgery. Br Dent J 223, 483–493 (2017). https://doi.org/10.1038/sj.bdj.2017.858
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.2017.858
This article is cited by
-
Simplifying differential diagnoses of orofacial conditions - a guide to surgical sieves and red flags
British Dental Journal (2021)