Abstract
Study design:
A retrospective analysis.
Objectives:
The objective of this study is to determine whether dipstick protein analysis (DSP) or random urine protein:creatinine ratios (UPC) are accurate in predicting clinical proteinuria in the chronic spinal cord injury (SCI) population.
Methods:
A retrospective analysis was performed in 219 veterans with SCI, comparing DSP and 24-h urine protein excretion. Sensitivity, specificity, predictive values (PV) and receiver–operator characteristic (ROC) curves of DSP in predicting clinical proteinuria were calculated with and without correction for specific gravity (SG). A prospective study was also performed in 62 SCI patients, comparing the UPC and 24-h urines. Sensitivity, specificity, PV and ROC curves of UPC in predicting clinical proteinuria were calculated.
Results:
Any level of positive DSP had high specificity, but low sensitivity, for detecting the presence of clinical proteinuria. ROC curves of DSP for identifying clinical proteinuria yielded area under the curve of 0.749 (95% confidence interval 0.699–0.794), and adjustment for SG did not significantly improve accuracy. A UPC of <0.3 was sensitive with a high negative PV for ruling out clinical proteinuria, whereas a ratio >0.8 was specific with a high positive PV. A UPC between 0.3–0.8 had an intermediate sensitivity and specificity.
Conclusion:
Urine collections of 24-h are still needed in the chronic SCI population for accurate detection of clinically significant proteinuria. DSP may not reliably detect low-grade clinical proteinuria, whereas a UPC below 0.3 may be used to rule out clinical range proteinuria.
Similar content being viewed by others
Introduction
Proteinuria is known to have both diagnostic and prognostic value in detection, confirmation and the monitoring of progression of renal disease.1, 2 Given the difficulty with 24-h urine collections, the National Kidney Foundation recommends measuring simple urine dipstick protein (DSP) for those at lower risk for kidney disease, or determining the random urine protein:creatinine ratio (UPC) for those at higher risk.3 DSP is accurate in predicting clinical proteinuria in the general population,4 and the use of the UPC to quantify proteinuria is considered accurate for normal individuals, pregnant women, patients with diabetes mellitus, renal transplant recipients, children with nephrotic syndrome5, 6, 7, 8 and patients with glomerulonephritis and impaired renal function.9
Proteinuria is recognized as an independent risk factor for cardiovascular disease,11 renal disease9 and increased mortality in the general population.11 The prevalence of clinical proteinuria (⩾0.5 g per day) in patients with chronic spinal cord injury (SCI) is greater than in the general population.10 The presence of clinical proteinuria is associated with increased cardiovascular and non-cardiovascular mortality in the SCI population.10
Protein excretion for 24-h is considered the gold standard for quantifying proteinuria in patients with chronic SCI; however, 24-h protein measurements have been shown to have significant variability on serial testing in these patients.13 Chronic SCI patients also have clinical characteristics that may decrease the accuracy of DSP and UPC in detecting and quantifying proteinuria. These factors include dilute urine4 and high rates of urinary tract inflammation, which may lead to increased levels of non-albuminuric proteins.14, 15 As urine dipsticks are more reactive to albumin than non-albumin proteins,16 dipstick analysis may not be as sensitive in predicting total proteinuria in patients with chronic SCI. Additionally, patients with chronic SCI often have decreased muscle mass with decreased creatinine production and excretion, which may lead to falsely elevated UPC ratios.17 The purpose of this study was to assess the accuracy of DSP and UPC in predicting clinical proteinuria in the SCI population.
Patients and methods
Patient and urine sample selection
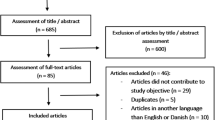
Computerized medical records of 219 patients with chronic SCI were reviewed at the Veterans Affairs Medical Center in Memphis, TN, USA. These patients had 24-h urine studies for both total protein excretion and creatinine clearance measurements as part of routine annual health maintenance.18 Samples were included if a 24-h urine collection and random urinalysis were performed within 48 h of each other. No samples were collected during an active urinary tract infection. To reduce potential collection bias, no more than two samples were collected from an individual patient. This led to the inclusion of 339 samples for study. Data collected from the 24-h urine included creatinine concentration (mg dl−1), protein concentration (mg dl−1) and volume. Data collected from the dipstick urinalysis included specific gravity (SG) and protein (negative, trace, 1+, 2+, 3+).
A prospective study was also performed in 62 inpatients with chronic SCI to evaluate the accuracy of the UPC in predicting clinical proteinuria. An early morning spot urine sample for UPC and a 24-h urine collection were performed for each patient under the direct observation of the research team. UPC was calculated as urinary protein (mg dl−1) divided by urinary creatinine (mg dl−1).
Clinical information obtained at the time of both studies included age, gender, ethnicity, type of injury, duration of injury and type of bladder management. Type of injury was defined as quadriplegia or paraplegia. Bladder management was one of four types: chronic indwelling Foley catheter, ileal conduit, intermittent catheterization or spontaneous voiding.
Data and statistical analysis
Levels of proteinuria of 24-h were used as the gold standard for defining the absence or presence of proteinuria. Clinical proteinuria was defined as ⩾0.5 gm per day.12 Sensitivity, specificity, positive predictive value (+PV), and negative PV (−PV) of DSP and UPC in predicting proteinuria were calculated. Receiver–operator characteristic (ROC) curves were created for DSP and UPC as screening tests for clinical proteinuria. Area under the curve (AUC) was calculated and P-values were considered significant if <0.05.
To assess the effect of urine concentration on the accuracy of DSP, a ROC curve was created using DSP adjusted for SG according to a previously published algorithm.4 Simply, for clinical proteinuria (⩾0.5 gm per day), all DSP levels were considered positive except for (a) trace proteinuria with a SG >1.015 and (b) 1+ proteinuria with a SG >1.025.
Sensitivity, specificity, PVs and ROC curves of UPC in predicting clinical proteinuria were calculated. The correlation between 24-h protein and morning spot UPC was analyzed using the concordance correlation coefficient (r) and linear regression. The level of agreement between morning UPC and 24-h protein was also assessed using the Bland–Altman method,19 both before and after excluding patients with massive proteinuria (>10 gm per day). Using this method, the mean difference between UPC and measured 24-h protein directly estimates the global bias. Bias represents the difference between an estimator's expectation and the true value of the parameter being estimated. The width of one s.d. of the mean difference between UPC and 24-h protein is an estimation of precision, defined as the closeness of agreement between independent test results obtained under stipulated conditions, with a large width indicating low precision.
To determine the potential effect of low creatinine excretion on the level of agreement between morning UPC and 24-h protein, additional Bland–Altman analysis was performed after categorizing the cohort by daily urine creatinine excretion rate (using 0.6 gm per day as a cut-off point). Statistical analysis was done using Med Calc Version 9.3.0.0 (Mariakerke, Belgium).
Results
Characteristics of the cohort
Table 1 shows the general characteristics of the retrospective and prospective cohorts of patients that were used to evaluate DSP and UPC as predictors for clinical proteinuria. The majority of patients were male, Caucasian, and paraplegic. Most either used a chronic indwelling Foley catheter or spontaneously voided.
DSP in predicting clinical proteinuria
Table 2 shows the sensitivities, specificities and PVs of DSP for 24-h proteinuria of 0.5 gm per day. Overall, a positive DSP of any level had a high specificity and +PV for the presence of ⩾0.5 gm per day of proteinuria. However, the sensitivity and −PV were low.
The mean and median SG values for DSP measures were 1.011 and 1.010, respectively. The percentage of false negative samples was higher in the dilute urines. For example, in the 90 patients with negative dipsticks and a SG ⩽1.005, 24 patients actually had clinical proteinuria. Negative and trace DSP failed to identify significant numbers of patients with clinical proteinuria.
Figure 1 shows the ROC curve for DSP as a screening test for clinical proteinuria. The AUC was 0.75 (95% confidence interval (CI) 0.70–0.79, P<0.001). Adjusting DSP for concentrated urines (i.e. classifying trace protein with a SG of 1.020 or greater and 1+ protein with a SG of 1.030, or greater as negative for clinical proteinuria) slightly improved the specificity of DSP for clinical proteinuria, but also worsened the sensitivity. The AUC decreased to 0.74 (95% CI 0.69–0.78, P<0.001) for the corrected dipstick (difference between AUC's=0.012, P=0.5). To evaluate the effect of dilute urine on the accuracy of DSP, a ROC curve was created for 0.5 g per day proteinuria, excluding urine specimens with a SG of <1.010 The AUC of the ROC curve showed only minimal improvement to 0.78 (95% CI 0.72–0.84, P<0.001), secondary to improvement in the sensitivity of the dipstick. When both concentrated and dilute urines were excluded, there were also minimal improvements in accuracy. AUC for the ROC improved from 0.75 to 0.80 (95% CI 0.75–0.85, P<0.001 with difference between AUC's=0.05, P=0.5 and sensitivity improving from 52.7 to 65.5%).
ROC curves for clinical proteinuria (0.5 g per day proteinuria), comparing DSP uncorrected (solid line) and corrected (dotted line) for SG. The curves nearly overlap, with an AUC of 0.749 (0.699–0.794, P<0.0001 compared with null hypothesis) for the uncorrected dipstick versus 0.737 (0.686–0.783, P<0.001) for the corrected dipstick (difference between AUC's=0.012, P=0.514).
UPC in predicting clinical proteinuria
Table 3 shows the sensitivity, specificity and PVs of UPC in predicting clinical proteinuria. The prevalence of clinical proteinuria in this cohort was 40.4%. A UPC ratio of <0.3 had a high sensitivity and high −PV for excluding clinical proteinuria. A UPC ratio of > 0.8 had a high specificity and high +PV for detecting clinical proteinuria. A UPC between 0.3 and 0.8 had an intermediate sensitivity and specificity for clinical significant proteinuria. Figure 2 shows the ROC curve of UPC as a screening test for clinical proteinuria. The AUC was 0.75 (95% CI 0.62–0.85, P<0.001).
ROC for UPC in detecting clinical proteinuria (0.5 g per day proteinuria). The best combination of sensitivity and specificity of UPC ratio for predicting clinical proteinuria occurs at an UPC cut-off point of 0.51, with sensitivity of 79.2% and specificity is 68.4% indicated by the box on ROC curve. AUC=0.75 (95% CI 0.624–0.852, P<0.0002).
There was a significant correlation between the UPC and the 24-h urine protein level (n=62, concordance correlation coefficient (r)=0.96, 95% CI 0.93–0.97, P<0.001), which is displayed by scatter plot in Figure 3. Excluding patients with 24-h urine protein >10 g per day (n=60), the concordance correlation coefficient (r) decreased to 0.45 (95% CI 0.23–0.64, P<0.001). The agreement between morning UPC and 24-h protein was assessed using the Bland–Altman method. The difference between the mean value and its related 95% CI showed an agreement between the UPC and 24-h protein levels (the bias was 1.19, and the 95% limits of agreements were between (−2.4 to −2.3). Exclusion of patients with massive proteinuria (⩾10 g per day) decreased the bias to −0.17 (95% limits of agreement −1.2 to −0.8; Figure 4). Although the agreement between the two methods improved after excluding patients with massive proteinuria (low bias and high precision) at levels of proteinuria <0.5 g per day, the level of agreement progressively decreased with increasing levels of proteinuria (increased bias and decreased precision; Figure 4).
Bland–Altman plot comparing the difference between the 24-h urine protein (g per day) and the UPC ratio sample versus the mean of the two methods with massive proteinuria (>10g per day) excluded. The dashed line represents the mean+2 s.d. The thick line represents the mean. The agreement between UPC and 24-h proteinuria is not uniform across the observed range of proteinuria. The agreement between the methods is quite good (low bias and high precision) at levels of proteinuria <0.5 g per day; whereas, the level of agreement progressively decreases with increasing levels of proteinuria (increased bias and decreased precision).
To determine the potential effect of low creatinine excretion on the level of agreement between morning UPC and 24-h protein, Bland–Altman analysis was also performed after excluding samples with <0.6 g creatinine per day. Exclusion of these samples did not improve either the global bias or precision (mean−2 s.d.=−2.4; mean+2 s.d.=2.6, Figure not shown), suggesting that the effect of low urine creatinine excretion on UPC was minimal.
Discussion
Accurate determination of urinary protein excretion in patients with chronic SCI is needed, as clinical proteinuria (>0.5 g per day) is predictive of cardiovascular, non-cardiovascular and all-cause mortality.10 The importance of proteinuria in the chronic SCI population is magnified by its higher prevalence rate, compared with the general population.10
The optimal method of measuring proteinuria in chronic SCI patients remains unclear. The measurement of 24-h urine protein is inconvenient and subject to collection errors. Evidence suggests that approximately 12–15% of 24-h urine collections are excluded from analysis because of errors during collection.6, 12 In the general population, measuring the urine creatinine as a function of body mass is used for evaluating the adequacy of 24-h urine samples. A similar, standardized method is not available for the chronic SCI population in whom creatinine excretion is highly variable, secondary to variable muscle mass. Benefits of 24-h collections include measurements of sodium and protein intake, and more accurate measurements of creatinine clearance in the chronic SCI population.20
Given the difficulty and limitations of 24-h samples, the National Kidney Foundation recommends random urine testing for proteinuria. In the general population, DSP has been shown to be a sensitive, but rather nonspecific test for both clinical proteinuria and microalbuminuria.4, 5 In our study, the opposite is true for SCI patients in whom DSP is very specific, but not sensitive for the detection of clinical proteinuria.
There are at least two possible explanations for the lack of sensitivity. The first is urine concentration. In the general population, the exclusion of concentrated urine samples, which may give falsely positive results, improves the specificity of DSP for clinical proteinuria.4 However, the specificity of DSP for clinical proteinuria was quite high in our SCI cohort. Our population overall had many very dilute urines, which led to a higher false negative rate of identifying clinical proteinuria. The second possible explanation is the type of protein detected by the dipstick method versus the 24-h total urine protein. SCI patients have a high rate of urinary tract inflammation and chronic pyelonephritis, which may lead to increased levels of non-albuminuric proteins.14, 15 As urine dipsticks are more reactive to albumin than non-albumin protein, urine dipsticks may be less sensitive in predicting total proteinuria in patients with SCI.
A UPC ratio of <0.3 had a high sensitivity and high −PV for excluding clinical proteinuria, and a UPC ratio of > 0.8 had a high specificity and high +PV for detecting clinical proteinuria. UPC between 0.3 and 0.8, however, had an intermediate sensitivity and specificity with significant false negative and false positive results. ROC curves showed ∼25% chance the UPC will lead to misclassification of clinical proteinuria. Bland–Altman analysis demonstrated excellent agreement between the UPC and 24-h proteinuria (low bias and high precision) at levels of proteinuria less than 0.5 g per day; however, global bias increased and precision decreased with progressive increments in proteinuria, thus, limiting the reliability of UPC for detecting clinical proteinuria. Exclusion of patients with reduced muscle mass, defined as urinary creatinine excretion <600 mg per day, did not improve the bias or precision of UPC, suggesting that reduced urinary creatinine excretion was not the primary reason for the discrepancies between UPC and 24-h proteinuria in detecting clinical range proteinuria. Given these limitations, UPC should not replace 24-h protein measurements for detecting clinically significant proteinuria in patients with SCI.
Limitations of the current study include the inherent limitations of 24-h urine collections, which were used as the ‘gold standard’ for quantifying proteinuria. In the prospective arm of the study, 24-h urine collections were performed under direct observation of the research team in an effort to minimize collection errors. The use of an all-male population and the single center study design may limit application to the general SCI population.
Conclusion
For the chronic SCI population, 24-h urine collections continue to be the preferred method for quantifying proteinuria. Routine dipstick urinalysis provides clinically relevant information for the SCI patient, but should not be used as the sole method to screen for clinical proteinuria, even after correction for SG. Spot UPC is of utility for ruling out clinical proteinuria at levels of <0.3 and in ruling in proteinuria at levels >0.8; however, intermediate levels (0.3–0.8) require confirmation with 24-h urine collections.
References
Ruggenenti P, Perna A, Mosconi L, Pisoni R, Remuzzi G . Urinary protein excretion rate is the best independent predictor of ESRD in non-diabetic proteinuric chronic nephropathies. Kidney Int 1998; 535: 1209–1216.
The ACE Inhibitors in Diabetic Nephropathy Trial Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? Ann Intern Med 2001; 134: 370–379.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2006; 39: S1–266.
Constantainer M, Sehgal AR, Humbert L, Constantainer D, Arce L, Sedor JR . A dipstick protein and specific gravity algorithim accurately predicts pathological proteinuria. Am J Kidney Dis 2005; 45: 833–841.
Rodby RA, Rohode RD, Sharon Z, Pohl MA, Bain RP, Lewis EJ . The urine protein to creatinine ratio as a predictor of 24-hr protein execretion in type 1 diabetic patients with nephropathy: The collaborative study group. Am J Kidney Dis 1995; 26: 904–909.
Gaspari F, Perico N, Remuzzi G . Timed urine collections are not needed to measure urine protein excretion in clinical practice. Am J Kidney Dis 2006; 47: 1–7.
Ramos JG, Martins-Costa SH, Mathias MM, Guerin YL, Barros EG . Urinary protein/creatinine ratio in hypertensive pregnant women. Hypertens Pregnancy 1999; 18: 209–218.
Dyson EE, Will EJ, Davison AM, O’Malley AH, Shepherd HT, Jones RG . Use of the urinary protein creatinine index to assess proteinuria in renal transplant patients. Nephrol Dial Trans 1992; 7: 450–452.
Morales JV, Weber R, Wagner MB, Barros EJ . Is morning urinary protein/creatinine ratio a reliable estimator of 24 h proteinuria in patients with glomerulonephritis and different levels of renal function? J Nephrol 2004; 17: 666–672.
Greenwell MW, Mangold TM, Tolley EA, Wall BM . Kidney disease as a predictor of mortality in chronic spinal cord injury. Am J Kidney Dis 2007; 49: 383–393.
Hillege HL, Fidler V, Diercks GFH . Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 2002; 106: 1777–1782.
Shidham G, Hebert LA . Timed urine collections are not needed to measure urine protein excretion in clinical practice. Am J Kidney Dis 2006; 47: 8–14.
Sepahpanah F, Burns SP, Mcknight B, Yang CC . Role of creatinine as a screening test in persons with spinal cord injury. Arch Phys Med Rehab 2006; 87: 524–528.
Menon EB, Tan ES . Pyuria Index of infection in patients with spinal cord injuries. Br J Urol 1992; 69: 144–146.
Bagley RS, Center SA, Lewis RM . The effect of experimental cystitis and iatrogenic blood contamination on the urine protein /creatinine ratio in dog. J Vet Intern Med 1991; 5: 66–70.
Bowie L, Smith S, Gochman N . Characteristics of binding between reagent strip indicators and urinary proteins. Clin Chem 1977; 23: 128–130.
Abitol C, Zilleruelo G, Freundlich M, Strauss J . Quantification of proteinuria with urinary protein/creatinine ratio and random testing with dipstick in nephrotic children. J Pediatr 1990; 116: 102–109.
Department of Veterans Affairs. Spinal cord injury services, Part XXIV Veterans Health Administration Manual M-. Department of Veterans Affairs: Washington (DC), 1994.
Bland JM, Altman DG . Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet 1995; 346: 1085–1087.
Mohler JL, Barton SD, Bloutin R A, Cowen DL, Flanigan RC . The evaluation of creatinine clearance in spinal cord injury patients. J Urol 1986; 136: 366–369.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Alshayeb, H., Gilless, J., Greenwell, M. et al. Determining the optimal method for proteinuria detection in chronic spinal cord injury. Spinal Cord 50, 153–158 (2012). https://doi.org/10.1038/sc.2011.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2011.89
Keywords
This article is cited by
-
Screening for proteinuria in ‘at-risk’ patients with spinal cord injuries: lessons learnt from failure
Patient Safety in Surgery (2014)