Abstract
Study design:
Prospective multicenter study.
Objective:
To clarify the significance of intramedullary Gd-DTPA enhancement in cervical myelopathy, the prevalence, morphologic features, clinical relevance and postoperative change were investigated.
Setting:
Four hospitals in Japan.
Methods:
A total of 683 patients with cervical myelopathy who underwent decompressive surgery were consecutively examined. T1, 2 and Gd-DTPA-enhanced MRI were taken before surgery. Fifty consecutive cases without intramedullary enhancement were allocated in the non-enhancement group. The following variables were investigated: prevalence of the enhancement, the morphologic feature, the relationship between the enhancement and T2 high-intensity areas, the change of the Japanese Orthopedic Association (JOA) score for cervical myelopathy and the change of the enhancement after surgery.
Results:
Intramedullary enhancement was observed in 50 cases (7.3%). The enhancements were observed between the most severely compressed disc and the cranial half of the lower vertebral body. On axial images, they were observed at the posterior or posterolateral periphery of the spinal cord. Enhancement areas were observed within T2 high-intensity areas and smaller than them. The preoperative JOA score was 9.8±2.8 points in the enhancement group and 9.8±3.3 points in the non-enhancement group (NS). The postoperative JOA score was 12.7±2.9 points in the enhancement group and 14.2±2.4 in the non-enhancement group (P=0.006). Intramedullary enhancement disappeared in 60% of the patients 1 year after surgery.
Conclusion:
Intramedullary enhancement indicated not the severity of preoperative symptoms, but a sign of a worse prognosis.
Similar content being viewed by others
Introduction
The signal intensity of the spinal cord on magnetic resonance imaging (MRI) is thought to reflect pathologic changes of the spinal cord and to be indicative of the clinical status in cervical myelopathy. There have been numerous studies concerning the relationship between MRI findings and the prognosis of cervical myelopathy.1, 2, 3, 4, 5 Although the signal changes in the spinal cord on T1-weighted images (T1WI) and T2-weighted images (T2WI) have been studied, there have been no systemic studies concerning Gadolinium–diethylenetriamine pentaacetic acid (Gd-DTPA) enhancement in cervical myelopathy.
The examination of Gd-DTPA enhancement in MRI provides more useful information in the assessment of intramedullary lesions. Intramedullary enhancement is known to appear in intramedullary tumors, multiple sclerosis, sarcoidosis, myelitis and spinal cord infarction. The intramedullary enhancement has been reported in the injured spinal cord occasionally6 and more rarely in cervical myelopathy.7, 8, 9 The recognition of the intramedullary enhancement in cervical myelopathy should help to distinguish it from other intramedullary lesions, while also providing useful knowledge for the treatment of the cervical myelopathy.
The purpose of this study was to reveal the prevalence, morphologic findings and clinical relevance of intramedullary enhancement in cervical myelopathy.
Patients and methods
This study was designed as a prospective multicenter study. Patients with cervical myelopathy who underwent Kurokawa's laminoplasty or anterior spinal fusion were consecutively investigated. However, the patients who disapproved the study, trauma-induced, a history of spinal surgery, rheumatoid arthritis, cerebral palsy and other brain disorders, Gd-DTPA hypersensitivity and claustrophobia were excluded. A total of 683 patients were enrolled in the study. The age of the patients was 63.1±13.0 (mean±s.d.) (95% confidence interval; 62.3–63.9 years) years. Sagittal T1, 2-weighted and Gd-DTPA-enhanced T1-weighted MRI and axial T2-weighted and Gd-DTPA-enhanced MRI were taken 1 month before surgery. A 1.0-Tesla superconducting imaging system (SMT-100, Shimadzu, Kyoto, Japan) or a 1.5-T superconducting imaging system (Magnetom H15, Siemens, Erlangen, Germany) were used for MRI. All patients who had intramedullary Gd-DTPA enhancement were referred to neurologists, or had their cerebrospinal fluid checked before surgery and the enhancements were confirmed as not caused by other diseases. Fifty consecutive patients without the intramedullary enhancement in one hospital were allocated in a non-enhancement group.
The following variables were investigated.
(1) Prevalence of intramedullary enhancement
(2) Duration of symptoms and the age at the surgery
(3) Location of intramedullary enhancement in the sagittal images and relationship with the compression
(4) Morphology of the enhancement area
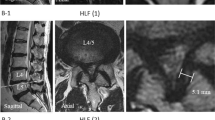
On sagittal-enhanced MR images, the shapes of the enhancement area were classified into two types. A short type was defined as that its length was within one vertebra length and a long type was longer than one vertebra length. On axial-enhanced MR images, the shapes of the enhancement area were classified into peripheral type, central type, scattering type, diffuse type, snake-eye type and nontypeable type (Figure 1). In the nontypeable type, the shape of the enhancement area could not be classified into any types, because all MR images were obtained in the same protocol and the axial image of the enhancement was not obtained clearly in some cases with very small enhancement.
(5) Signal intensity on T1WI and T2WI and the relationship between the Gd-DTPA enhancement and high signal intensity area on T2WI
Signal intensities of the spinal cord were evaluated on T1WI and T2WI. On sagittal and axial T2WI, the shape of the high signal intensity area was classified in the same way as the Gd-DTPA enhancement area.
(6) Change in the Japanese Orthopedic Association (JOA) score for cervical myelopathy one year after surgery and the recovery rate
The JOA score quantified symptom and neurological impairment.10 The JOA scores were measured before and 1 year after surgery. The recovery rate was evaluated using the formula proposed by Hirabayashi et al.10: recovery rate (%)=((postoperative JOA Score)−(preoperative JOA Score))/(17−(preoperative JOA Score)) × 100.
(7) Change of the Gd-DTPA enhancement after surgery
Forty patients with intramedullary enhancement before surgery agreed to the second MRI examination 1 year after surgery. The MRI was performed according to the same protocol as the first examination. The relationship between the change of the enhancement and the recovery rate was investigated.
We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during the course of this research.
Statistical analysis
The data are expressed as the means±s.d. and 95% confidence interval (CI). Statistical differences in the duration of symptoms and age were tested by the unpaired Student's t-test and checked by the Mann–Whitney U-test. Differences in the JOA score and recovery rate were tested by the Mann–Whitney U-test. Differences in the JOA scores for each morphological type of intramedullary enhancement were analyzed using the one-way ANOVA with the post-test and Tukey–Kramer Multiple Comparison test. A probability value of less than 0.05 was considered to indicate statistical significance. All tests were two-tailed. All statistical analyses were performed using a personal computer with the commercially available computer software program (GraphPad InStat 3.0 for Windows, GraphPad Software, San Diego CA, USA).
Results
Prevalence of intramedullary Gd-DTPA enhancement
Intramedullary enhancement was observed in 50 cases (7 3%) out of 683 cases of cervical myelopathy.
Duration of symptoms and the age at surgery
The duration of symptoms was 13.4±19.3 (95% CI; 7.9–19.0) months in the enhancement group and 16.7±21.9 (95% CI; 10.4–22.8) months in the non-enhancement group. There was no significant difference between them. The mean age of the patients was 57.7±12.7 (95% CI; 54.1–61.3) years in the enhancement group and 62.6±11.8 (95% CI; 59.3–66.0) years in the non-enhancement group. The patients in the enhancement group were significantly younger than the other patients enrolled in the study (P=0.005), and the patients in the non-enhancement group (P=0.046).
Location of intramedullary Gd-DTPA enhancement in the sagittal images and the relationship with the compression
All intramedullary enhancement areas were observed at the most severely compressed intervertebral disc level, which was defined as the disc level of the maximally compressed spinal cord. Intramedullary enhancements were observed in seven cases (14%) at the C3/4 level, 18 cases (36%) at the C4/5, 21 cases (42%) at the C5/6 and four cases (8%) at the C6/7. The most severe compression in the non-enhancement group were observed in 10 cases (20%) at the C3/4 level, 21 cases (42%) at the C4/5, 16 cases (32%) at the C5/6 and three cases (6%) at the C6/7. There were no significant differences in the most severe compressed level between the enhancement and non-enhancement groups.
Morphology of the Gd-DTPA enhancement area
All cases showed the short type on sagittal images. Enhancements were observed between the intervertebral disc and the cranial half of the lower vertebral body in most cases (Figure 2). On axial images, 23 cases (46%) were classified as the peripheral type, four cases (8%) as central type and 15 cases (30%) as scattering type. There were no diffuse type and snake-eye type. Eight cases (16%) were classified as nontypeable type. Gd-DTPA enhancements were observed in the posterior or posterolateral peripheral area in the spinal cord in many cases.
On sagittal-enhanced MR images, the lengths were within one vertebra length in all cases. Enhancements were observed between the most severely compressed intervertebral disc and the cranial half of the lower vertebral body (a, c, e). On axial images, the enhancements were classified into peripheral type (b), central type (d) and scattering type (f). Arrows indicate Gd-DTPA enhancement areas.
Signal intensity on T1WI and T2WI and relationship between Gd-DTPA enhancement area and high signal intensity area on T2WI
On sagittal images of T1WI, all cases showed iso signal intensity in comparison with the normal spinal cord in both groups. On sagittal images of T2WI, intramedullary high signal intensity areas were observed in all cases (100%) in the enhancement group and in 48 cases out of 50 cases (96%) in the non-enhancement group.
Regarding the morphology of T2 high signal intensity area on the sagittal images, 24 cases (48%) showed the short type and 26 cases (52%) showed the long type in the enhancement group, whereas, 32 cases (67%) showed the short type and 16 cases (33%) showed the long type in the 48 non-enhancement cases.
On the axial images, the transverse shapes of the T2 high signal intensity areas are shown in Figure 3. Thirty-eight cases (76%) showed the scattering type or diffuse type in the enhancement group, whereas, the snake-eye type was the most frequent in the non-enhancement group. The T2 high signal intensity area in the enhancement group presented with distinctive large and clear signal intensity in comparison with the non-enhancement group. Enhancement areas were included within the T2 high signal intensity areas and smaller than those in all cases (Figure 4). In the cases with the peripheral type and snake-eye type of T2 high signal intensity areas, few intramedullary enhancements were observed.
Shape of T2 high signal intensity in the enhancement and non-enhancement group. In the enhancement group, 38 cases (76%) showed the scattering type or diffuse type T2 high signal intensity area, whereas in the non-enhancement group, the snake-eye type T2 high signal intensity area was 35% and the most frequent.
Change of JOA score for cervical myelopathy and the recovery rate
The preoperative JOA score was 9.8±2.8 (95% CI; 9.0–10.6) points in the enhancement group and 9.8±3.3 (95% CI; 8.9–10.7) points in the non-enhancement group. There was no significant difference in preoperative severity presented by the JOA score between the enhancement group and non-enhancement groups. The preoperative JOA score was 9.6±2.7 (95% CI; 8.4–10.7) points in the peripheral type, 7.5±2.4 (95% CI; 3.7–11.3) points in the central type, 11.2±2.6 (95% CI; 9.8–12.6) points in the scattering type and 9.0±2.7 (95% CI; 6.7–11.3) points in the nontypeable type. The JOA score of the central type was lower than that of other types. However, no significant differences were observed in the JOA scores among the morphological types of intramedullary enhancement. The postoperative JOA score was 12.7±2.9 (95% CI; 11.8–13.7) points in the enhancement group and 14.2±2.4 (95% CI; 13.6–14.1) in the non-enhancement group (Figure 5). The recovery rate was 42.0±28.5% (95% CI; 32.9–51.2) in the enhancement group and 61.3±25.9% (95% CI; 53.9–68.6) in the non-enhancement group. The postoperative JOA score (P=0.006) and recovery rate (P=0.002) in the enhancement group were significantly lower than those in the non-enhancement group.
Change of the Gd-DTPA enhancement after surgery
In 24 cases out of the 40 cases (60%), the enhancement disappeared. In 15 cases, the enhancement still remained, but decreased in area and intensity. There was no significant difference in the recovery rate between the cases in which the enhancement disappeared and the cases in which the enhancement decreased.
In one case, the enhancement area enlarged (Figure 6). In a 69-year-old female with cervical spondylotic myelopathy, the intramedullary enhancement at C4/5 was observed in an enhanced MRI before surgery. Her symptoms were improved immediately after Kurokawa's laminoplasty. However, they became aggravated 6 months later without any specific cause. The JOA score aggravated from 5 points to 4 points 1 year after surgery. MR images showed extensive swelling of the spinal cord and enlargement of the intramedullary enhancement. The type of the enhancement area on axial image changed from a peripheral type to a diffuse type.
Enhanced MRI obtained before surgery showed the intramedullary Gd-DTPA enhancement at C4/5 (a, c). The enhanced MRI 1 year after surgery showed the extensive swelling of the spinal cord and the enlargement of the intramedullary Gd-DTPA enhancement (b, d). The type of Gd-DTPA enhancement area on axial image changed from peripheral type to diffuse type. Arrows indicate Gd-DTPA enhancement areas.
Discussion
There have been a few reports addressing the intramedullary Gd-DTPA enhancement in cervical myelopathy. Sato et al.7 investigated Gd-DTPA enhancement in 53 cases of cervical spondylotic myelopathy. Diffuse enhancements were observed at most severely compressed intervertebral disc level in nine cases. The enhancement disappeared in three cases and reduced in six cases 1 year after surgery. Cabraja et al.11 reported a case of cervical myelopathy. MR images showed an atypically enlarged intramedullary T2 high intensity with enhancement that resembled an intramedullary tumor. As there have been no systemic studies regarding the intramedullary enhancement, the prevalence of the enhancement was not revealed in cervical myelopathy. In this study, the intramedullary enhancement was observed in 7.3%, whereas T2 high signal intensity areas were observed in all enhancement cases and in 96% in the non-enhancement cases. In comparison with the prevalence of the T2 high signal intensity, intramedullary enhancement was uncommon.
Intramedullary enhancement is known to appear in intramedullary lesions such as intramedullary tumors, multiple sclerosis, sarcoidosis, myelitis and spinal cord infarction. The recognition of the imaging feature should help in distinguishing the cervical myelopathy with the intramedullary enhancement from the other intramedullary lesions. Intramedullary tumors tend to enhance in a central area, whereas demyelinating disease or sarcoidosis show a more peripheral enhancement. The enhancement in multiple sclerosis correlates with the clinical development of the disease. The multiple sclerosis is often initially enhanced and thereafter undergoes a non-enhancing state. Spinal cord infarction typically reveals a diffuse enhancement 10 to 21 days after infarction. In this study, the enhancements were observed between the intervertebral disc and the cranial half of the lower vertebral body and within one vertebra length on sagittal images. On axial images, they showed the peripheral type or scattering type in many cases. These morphological features provide important information to distinguish cervical myelopathy from other intramedullary lesions.
Various factors such as the preoperative duration, the severity of symptoms and the transverse area and the antero-posterior compression ratio of the spinal cord have been reported to influence the prognosis in cervical myelopathy.4, 12 Although the clinical relevance of signal intensity changes on T1WI and T2WI in cervical myelopathy has been widely studied, it has remained controversial.1, 2, 3, 10 Mizuno et al.4 described that the snake-eye T2 high signal intensity indicated an irreversible necrosis and showed a worse outcome. In this study, the snake-eye T2 high signal intensity was observed in 4% of the enhancement group and 35% in the non-enhancement group. The snake-eye T2 high signal intensity was observed commonly in cervical myelopathy and did not indicate bad prognosis. On the other hand, the intramedullary enhancement indicated a worse prognosis in this study. The intramedullary enhancement on preoperative MRI should be a convincing predictor of the surgical outcome in cervical myelopathy.
Although the mechanism of intramedullary enhancement in spinal cord injury (SCI) and cervical myelopathy is not clear, there are a few speculations with reference to the pathological mechanism of the intramedullary enhancement in the SCI.6, 13 The Gd-DTPA enhancement indicates a disruption of the spinal cord parenchyma and a disturbance of the blood-cord barrier (BCB) in the injured spinal cord. During the early phase of the neovascularization in the gliosis after SCI, the BCB is incomplete and induces the enhancement.6 In addition, the disturbed venous circulation caused by SCI resulted in local venous hypertension at the affected level.14 Such venous hypertension may thus eventually result in the hyperpermeability of the intramedullary vessels and thereby cause intramedullary enhancement. In this study, 98% of the enhancement in cervical myelopathy either disappeared or decreased in intensity of the enhancement after surgery. This suggests that chronic compression may therefore lead to a disturbance of BCB and venous hypertension, which thus causes the enhancement, and such enhancement thereafter either disappears or decreases in intensity because of the improvement of the pathologic conditions as a result of the surgery.
References
Morio Y, Yamamoto K, Kuranobu K, Murata M, Tuda K . Does increased signal intensity of the spinal cord on MR images due to cervical myelopathy predict prognosis? Arch Orthop Trauma Surg 1994; 113: 254–259.
Matsumoto M, Toyama Y, Ishikawa M, Chiba K, Suzuki N, Fujimura Y . Increased signal intensity of the spinal cord on magnetic resonance images in cervical compressive myelopathy? Does it predict the outcome of conservative treatment? Spine 2000; 25: 677–682.
Chen CJ, Lyu RK, Lee ST, Wong YC, Wang LJ . Intramedullary high signal intensity on T2-weighted MR images in cervical spondylotic myelopathy: predictors of prognosis with type of intensity. Radiology 2001; 221: 789–794.
Mizuno J, Nakagawa H, Inoue T, Hashizume Y . Clinicopathological study of ‘snake-eye appearance’ in compressive myelopathy of the cervical spinal cord. J Neurosurg 2003; 99 (Suppl 2): 162–168.
Mastronardi L, Elsawaf A, Roperto R, Bozzao A, Caroli M, Ferrante M et al. Prognostic relevance of the postoperative evolution of intramedullary spinal cord changes in signal intensity on magnetic resonance imaging after anterior decompression for cervical spondylotic myelopathy. J Neurosurg Spine 2007; 7: 615–622.
Terae S, Takahashi C, Abe S, Kikuchi Y, Miyasaka K . Gd-DTPA-enhanced MR imaging of injured spinal cord. Clin Imaging 1997; 21: 82–89.
Sato T, Kojima T, Onuma H, Hashimoto M, Kokubun S, Hyodo H . Intramedullary enhanced lesion in MRI of cervical spondylotic myelopathy. In Japanese Seisaigeka 1993; 36: 917–922.
Nagashima H, Morio Y, Teshima R . Re-aggravation of myelopathy due to intramedullary lesion with spinal cord enlargement after posterior decompression for cervical spondylotic myelopathy: serial magnetic resonance evaluation. Spinal Cord 2002; 40: 137–141.
Boet R, Chan YL, King A, Mok CT, Poon WS . Contrast enhancement of the spinal cord in a patient with cervical spondylotic myelopathy. J Clin Neurosci 2004; 11: 512–514.
Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K . Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine 1981; 6: 354–364.
Cabraja M, Abbushi A, Costa-Blechschmidt C, van Landeghem FK, Hoffmann KT, Woiciechowsky C et al. Atypical cervical spondylotic myelopathy mimicking intramedullary tumor. Spine 2008; 15: E183–E187.
Fujiwara K, Yonenobu K, Ebara S, Yamashita K, Ono K . The prognosis of surgery for cervical compression myelopathy. An analysis of the factors involved. J Bone Joint Surg Br 1989; 71: 393–398.
Jellinger K . Neuropathology of cord injuries. In: Vinken PJ, Bruyn GW (eds). Handbook of Clinical Neurology, vol 25 Injuries of the Spine and Spinal Cord. North Holland: Amsterdam, 1976, pp 43–121.
Lee J, Koyanagi I, Hida K, Seki T, Iwasaki Y, Mitsumori K . Spinal cord edema: unusual magnetic resonance imaging findings in cervical spondylosis. J Neurosurg 2003; 99 (Suppl 1): 8–13.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ozawa, H., Sato, T., Hyodo, H. et al. Clinical significance of intramedullary Gd-DTPA enhancement in cervical myelopathy. Spinal Cord 48, 415–422 (2010). https://doi.org/10.1038/sc.2009.152
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.152
Keywords
This article is cited by
-
Blood-spinal cord barrier disruption in degenerative cervical myelopathy
Fluids and Barriers of the CNS (2023)
-
The imaging of cervical spondylotic myeloradiculopathy
Skeletal Radiology (2023)
-
Degenerative cervical myelopathy — update and future directions
Nature Reviews Neurology (2020)
-
Patients with degenerative cervical myelopathy have signs of blood spinal cord barrier disruption, and its magnitude correlates with myelopathy severity: a prospective comparative cohort study
European Spine Journal (2020)
-
Spinal cord swelling in patients with cervical compression myelopathy
BMC Musculoskeletal Disorders (2019)