Abstract
Study design:
Prospective longitudinal study.
Objectives:
The aim of this study was to examine the effects of transcranial magnetic stimulation (TMS) on the soleus H reflex in patients with spinal cord injury (SCI) before and after locomotion training.
Setting:
Neurorehabilitation hospital in Barcelona, Spain.
Methods:
H reflex was elicited in 29 incomplete patients with SCI at 20, 50 and 80 ms after single vertex TMS, and compared with 13 healthy subjects. Patients were subdivided in two groups according to time since injury (<3 months, 3–12 months), and all received training with electromechanical systems. The H reflex modulation pattern to TMS was reassessed and the results were analyzed as a function of change in the patient clinical score.
Results:
Healthy subjects showed a significant H reflex facilitation at 20 ms (186.1%) and at 80 ms (190.6%) compared with the control H reflex. In patients, the H reflex facilitation at 20 ms was significantly reduced before training (142.5%, P=0.039) compared with healthy subjects. After training, patients with <3 months exhibited an increase in H reflex facilitation at 20 ms (170.7%, P=0.04), a greater gait velocity (P=0.014) and a positive correlation with the walking index for spinal cord injury (WISCI II) scale (P=0.050), compared with those with >3 months.
Conclusions:
TMS-induced H reflex modulation may help in the assessment of changes in the descending control of leg reflexes. Our results suggest that the changes on reflex modulation in patients with SCI occur within the first 3 months after injury.
Similar content being viewed by others
Introduction
The modulation of reflexes is an important aspect of motor control that implies timely and appropriate traveling of signals and commands in ascending and descending tracts of the spinal cord. Consequently, patients with spinal cord injury (SCI) have difficulties in the control of lower limb movements for performing purposeful acts such as walking.
Incomplete patients with SCI have the potential to regain some form of ambulatory function, predominantly at the early phase after injury.1 On the basis of previous animal models, Barbeau et al.2 described a system for locomotor rehabilitation using a treadmill, body weight support and manual assistance. This type of training has been considered as an effective method for improving the walking ability of patients with SCI.3 To provide electromechanically assisted locomotion, new systems have appeared on the market, such as the Lokomat4 and the Gait Trainer GT I5 showing improvement in gait of patients with incomplete SCI.6 However, there is lack of physiological measures correlating with such improvements.
The ankle jerk and its electrophysiological counterpart, the soleus H reflex,7 are responses of reflex circuits functionally engaged in gait control. The size of the H reflex is modified by changes in body posture,8 walking9 and other maneuvers (for review, see Misiaszek10). When preceded by a transcranial magnetic stimulation (TMS), the H reflex may show an early inhibition,11 and two facilitatory phases: an early phase peaking between 10 and 20 ms, and a late phase, peaking at about 80 ms.11, 12, 13
Such a modulation pattern may reflect the activity in functionally relevant spinal cord circuits and, therefore, it may be abnormal in people with SCI. Gait-related phasic modulation of the H reflex does not always show abnormalities in people with chronic SCI.14 However, we expected that patients with SCI will present with an abnormal pattern of TMS-induced H reflex modulation that could be modified along with improvement in gait capabilities. If such change occurs, it is expected to be more prominent at early than at late phases of the recovery process after SCI. Therefore, we studied the pattern of TMS-induced modulation of the soleus H reflex in two groups of patients with incomplete motor SCI: patients with less than 3 months since injury and patients whose injury occurred between 3 months and 1 year.
Patients and methods
Subjects
We studied 29 patients with motor incomplete SCI (24 men and 5 women, mean age 47 years). Patients were divided in two groups. Group A included 16 patients with less than 3 months after injury (mean 1.84 months, s.e. 0.20). Group B included 13 patients whose injury occurred between 3 months and 1 year (mean 7.93 months, s.e. 2.55). Demographic and clinical data are shown in Table 1. Reference data were gathered from a group of 13 healthy volunteers, 10 men and 3 women, with a mean age of 32 years. Both control subjects and patients gave their informed consent for the study, which was approved by the institutional review board.
Clinical and functional assessment
Main outcome measures, collected at the beginning and end of the study, as follows: (1) lower extremities motor score (LEMS) obtained from the standardized American Spinal Injury Association (ASIA) clinical examination15; (2) walking index for spinal cord injury (WISCI II) scale16 to quantify patient's walking ability; (3) ten-meter walking test (10MWT) for gait velocity assessment.
Gait training program
Patients used the Lokomat or the Gait Trainer GT 1 depending on the availability of the systems. The starting time of the training was determined by the time that patients spent at other hospitals before being transferred to our center. They received training on one of the systems daily, 5 days per week for 8 weeks. The sessions started with 20 min, progressing as tolerated up to 45 min.
H reflex recording and TMS stimulation
Subjects were sitting in the wheelchair, with their right foot resting on a footrest allowing for a stable knee position at an angle of 60°. H reflexes were evoked in the right soleus by electrical stimulation of the posterior tibial nerve. The stimulating electrodes were attached at the popliteal fossa, over the course of the nerve, with the cathode proximal. Stimulus duration was 1 ms. The intensity was adjusted to obtain an H reflex of about 1 mV amplitude with minimal or no concurrent M response. In all subjects, we made sure that this amplitude ranged between 10 and 15% of the M wave obtained with the same recording to supramaximal electrical stimulation. Whenever a small M wave was obtained concurrently with the H reflex, we checked if its size modified along the experiment. If this was the case, we excluded those trials from the analysis and repeated the test. The recording electrode was a bipolar surface electrode with a fixed distance of 2 cm between active and reference, firmly attached to the posterior leg, over the soleus muscle.
For TMS we used a Magstim Super Rapid stimulator (Magstim, Whitland, UK), equipped with a double cone coil, set up to deliver single pulses time-locked to the posterior tibial nerve stimulation. The coil was fixed with an elastic band over the head, in the best position for eliciting a stable motor-evoked potential (MEP) in soleus. In control subjects, we used an intensity of TMS 90% below threshold for the MEP in the soleus. In patients, we first tried to induce an MEP with up to maximum output intensity. Since no MEPs were obtained in any patient, we decided to set up the TMS at a fixed intensity of 10% above the mean threshold for control subjects. The same intensity was applied in both situations (before and after the gait training).
Procedure
All recordings were carried out with a Medelec Synergy electromyograph (Oxford Instruments, Surrey, UK), using a band-pass frequency filter between 20 and 5000 Hz, a gain of 1000 μV cm−1 and a sweep duration of 500 ms. Recordings were digitized and stored for off-line analysis.
We first collected five H reflexes to single posterior tibial nerve stimuli, which were used as the control values. Then we applied the same stimuli preceded by TMS at 20, 50 or 80 ms interstimulus intervals (test trials). These intervals were chosen in view of their relevance for definition of the pattern of TMS modulation of the H reflex.12, 14 We collected five responses at each interval. The intertrial interval was at least 10 s to prevent carryover effects. Interstimuli intervals were randomly mixed. Also, a few control trials were intermingled with the test trials to control for possible baseline shifts in the amplitude of the control H reflex. If that was the case, we made slight adjustments to the stimulus intensity to keep the H reflex control at about 1 mV amplitude along the whole session.
The experiments were performed once in the group of healthy subjects, and before and after the gait training in the patients group (Figure 1). The fact that all patients were included in the gait training program prevented us to examine the effects of such intervention on eventual clinical and neurophysiological changes. However, we considered that a tendency to homogenous outcome would speak in favor of an effect of gait training, whereas differences between the two groups would suggest no relevant effect of gait training.
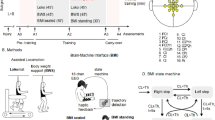
Experimental procedure. Baseline H reflex was obtained applying the electrical stimulation (ES) to the posterior tibial nerve. To induce a modulation of the H reflex, we used cortical transcranial magnetic stimulation (TMS). We studied the modulation of the H reflex when the ES was applied at 20, 50 and 80 ms after the TMS.
Data reduction and analysis
At baseline and at all intervals we measured the peak-to-peak amplitude of the H reflex with peak detection software (Matlab, Natick, MA, USA). In both, healthy subjects and people with an SCI, the mean value of the amplitude of the H reflex obtained in control trials was assigned 100%, and the amplitude of the H reflexes obtained from the study at the different intervals was expressed as its percentage value. The results are presented as means along with the standard error of the mean.
In this way, we obtained normalized data for group comparison, avoiding interindividual variability. One-way analysis of variance was used to determine the interval at which the H reflex amplitude was significantly different between control and test trials for each group. We also examined whether the effects of TMS on the H reflex were different in healthy subjects and patients at each interval tested. For this, we used the t-test to compare the H reflex amplitude change, expressed in percentage of the mean obtained in control trials, between healthy subjects and patients. Statistical significance was set at P=0.05.
Results
All subjects tolerated well the study and completed the procedure. At baseline, patients had an abnormally reduced LEMS, WISCI and gait velocity. The two SCI groups were homogeneous in all clinical and functional variables before start of training (Table 1). No differences were present in the size of the supramaximal M wave or the size of the H reflex in control trials between healthy subjects and patients of the two groups.
H reflex modulation by TMS in patients before training compared with healthy subjects
In healthy subjects, TMS induced a significant H reflex facilitation at 20 ms (early phase, 186.1±17.8%) and at 80 ms (late phase, 190.6±15.1%), but not at 50 ms (135.9±14.5%).
In patients with SCI, the H reflex was significantly larger when modulated by TMS at the three intervals than at baseline without TMS (P<0.05). However, such facilitation did not follow the pattern found in healthy subjects because of significantly reduced facilitation at the interval of 20 ms in the SCI group (142.5±7.8%, P=0.039) with respect to healthy subjects. There was a reduced facilitation also at the interval of 80 ms (160.3±10.8%) that was not significantly different from control subjects. Examples of the recordings are given in Figure 2 and the results of all subjects are represented in Figure 3.
Example of H reflex modulation by transcranial magnetic stimulation (TMS). Once the H reflex was obtained, the combined TMS/electrical stimulation (ES) was applied at different intervals between each other obtaining a modulation of the H reflex. This modulation was different in controls compared with spinal cord injury (SCI) subjects, observing a greater H reflex facilitation in the control group at the early and late phases. Single sweeps are shown superimposed.
H reflex modulation by transcranial magnetic stimulation (TMS) before training in all groups of subjects. Both the control subjects and the patients exhibited significant facilitation of the H reflex with respect to baseline (100%) at all intervals tested except for the interval of 50 ms in control subjects. Facilitation at the interval of 20 ms was significantly reduced in the spinal cord injury group with respect to control subjects (asterisk).
Changes in the H reflex modulation by TMS in the two groups of SCI subjects before and after gait training
After training, there was a significantly greater facilitation of the H reflex at the early phase (P=0.04) on subjects of group A (170.7±10.2%), compared with those of group B (125.3±5.6%) (Figure 4). No significant modifications were observed in the effects of TMS at intervals of 50 and 80 ms.
H reflex modulation by transcranial magnetic stimulation in spinal cord injury subjects before and after training. Before training, the H reflex facilitation was not different between groups A (less than 3 months after injury) and B (more than 3 months after injury). After training, H reflex facilitation at 20 ms was significantly greater in group A than in group B (asterisk). No other differences were found between groups A and B before or after training.
Clinical and functional parameters (LEMS, WISCI II and 10MWT)
In general, all parameters improved after the gait training period (Figure 5). We found significant differences in the outcomes studied before and after training: LEMS (P=0.003, group A; P=0.001, group B), WISCI (P=0.001, group A; P=0.008, group B) and velocity (P=0.002, group A; P=0.016, group B). The percentage change in gait velocity after training was significantly greater in group A than in group B (P=0.014).
Lower extremities motor score (LEMS), walking index for spinal cord injury (WISCI) and gait velocity changes after training. Mean and standard error of the mean for the LEMS, WISCI and velocity in patients before and after gait training for group A (less than 3 months after injury) and group B (more than 3 months after injury). A significant increase in LEMS, WISCI and velocity was found after training for both groups of subjects (P<0.05) but the percentage increase in velocity was significantly greater in group A than in group B (P=0.014).
Correlation between clinical improvement and H reflex modulation by TMS
Because the early phase (20 ms) of H reflex modulation was the one showing a larger increase after gait training, we analyzed whether this change was correlated with improvement in clinical outcomes in the two groups of patients separately. There was a statistically significant positive correlation between the improvement in WISCI and early H reflex facilitation in patients of group A (P=0.050), whereas no other significant correlations were found (Figure 6).
Correlation between walking index for spinal cord injury (WISCI) and H reflex facilitation by transcranial magnetic stimulation at the interval of 20 ms after training in subjects with less than 3 months after injury. The improvement in WISCI scale after the training was related to a greater percentage of H reflex facilitation (P=0.050).
Six patients had no change in the functional outcomes after the training but showed a slight increase in the H reflex facilitation (40.39±14.4%).
ASIA, SCI etiology, electromechanical system and H reflex modulation by TMS
As to the type of incomplete motor SCI (ASIA C vs D), etiology of the SCI and type of electromechanical system used, we found no differences at any of the intervals studied before and after the gait training.
Discussion
Our results have furnished three relevant conclusions: (1) patients with incomplete motor SCI have an abnormal TMS-induced modulation of the soleus H reflex; (2) the H reflex facilitation at the interval of 20 ms increased in all patients, but the percentage increase was significantly larger in patients within 3 months than in patients with longer time after injury; (3) there was a good correlation between the increase of H reflex facilitation and the improvement in functional aspects after 8 weeks of training in the group with less than 3 months after injury.
In healthy subjects, the H reflex amplitude modulation by TMS shows an early phase, peaking at 10–30 ms, and a late phase, peaking at 60–130 ms.12, 13, 14 According to Valls-Solé and co-workers,13 the first phase may result from the generation of an excitatory postsynaptic potential (EPSP) in the α-motoneuron, whereas the mechanisms responsible for the second phase are not clear. In a study of patients with SCI, Wolfe et al.17 reported the absence of modulation in patients with complete motor lesions, and a partial facilitation present on incomplete motor lesions (ASIA C and D). The results in our patients at baseline are comparable with those reported in incomplete motor lesions by Wolfe et al.17 and suggest an effective TMS-induced motoneuronal EPSP. Furthermore, the pattern of TMS-induced H reflex modulation changed more in patients with shorter time after injury. Interestingly, the change occurred mainly in the early phase (20 ms). We assume that this increase in facilitation is due to the arrival of a more effective and probably larger EPSP to the spinal cord motoneurons innervating leg muscles with progression of recovery.
A few patients with no improvement in the functional outcome measures exhibited a slight increase in the H reflex facilitation in the follow-up examination. A possible explanation for this observation is that gait training would induce a subclinical change in H reflex modulation. Because all our patients were engaged in some form of gait training, we cannot know the role of gait training in the changes described. We do not know, either, if another form of rehabilitation or even no rehabilitation at all would have led to the same effect or not. However, the fact that no significant changes of the H reflex modulation were seen in the follow-up examination in patients that had the SCI more than 3 months before the study, suggests that time after injury might be the most important variable to take into account for plasticity changes in the spinal cord that lead to better modulation of the H reflex.
In terms of neural tracts involved in this process, the fast corticospinal tract seems to mediate the early phase of facilitation, as previously described by Wolfe et al.17 The origin of the late facilitation is less clear, with different hypothesis: corticobulbospinal projections,18 peripheral afferent inputs19 or a summation of corticospinal, brainstem and peripheral influences.20
Patients that were included in the study within 3 months after the SCI had different outcomes defined by clinical improvement. Patients with better clinical outcome had a larger facilitation of the H reflex, with a statistically significant positive correlation with the WISCI scale score. This is an important aspect adding evidence from the neurophysiological point of view to the importance of the early training after SCI.
The H reflex modulation by TMS may be an interesting clinical neurophysiological tool to provide quantified measures to the supraspinal control of lower limb reflexes in patients with SCI. Its correlation with improvement in gait abilities during the first 3 months after injury suggests that it may have clinical applicability.
References
Waters RL, Adkins RH, Yakura JS, Sie I . Motor and sensory recovery following incomplete tetraplegia. Arch Phys Med Rehabil 1994; 75: 306–311.
Barbeau H, Wainberg M, Finch L . Description and application of a system for locomotor rehabilitation. Med Biol Eng Compt 1987; 25: 341–344.
Wernig A, Müller S, Nanassy A, Cagol E . Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injury persons. Eur J Neurosci 1995; 7: 823–829.
Colombo G, Joerg M, Schreider R, Dietz V . Treadmill training of paraplegic patients using a robotic orthosis. J Rehabil Res Dev 2000; 37: 693–700.
Hesse S, Uhlenbrock D . A mechanized gait trainer for restoration of gait. J Rehab Res Dev 2000; 37: 701–708.
Wirz M, Zemon DH, Rupp R, Scheel A, Colombo G, Dietz V et al. Effectiveness of automated locomotor training in patients with chronic incomplete spinal cord injury: a multicenter trial. Arch Phys Med Rehabil 2005; 86: 672–680.
Schieppati M . The Hoffmann reflex: a means of assessing spinal reflex excitability and its descending control in man. Prog Neurobiol 1987; 28: 345–376.
Goulart F, Valls-Solé J, Alvarez R . Posture-related changes of soleus H-reflex excitability. Muscle Nerve 2000; 23: 925–932.
Phadke CP, Wu SS, Thompson FJ, Behrman AL . Comparison of soleus H-reflex modulation after incomplete spinal cord injury in 2 walking environments: treadmill with body weight support and overground. Arch Phys Med Rehabil 2007; 88: 1606–1613.
Misiaszek JE . The H-reflex as a tool in neurophysiology: its limitations and uses in understanding nervous system function. Muscle Nerve 2003; 28: 144–160.
Nielsen J, Petersen N . Evidence favouring different descending pathways to soleus motoneurones activated by magnetic brain stimulation in man. J Physiol 1995; 486.3: 779–788.
Valls-Solé J, Valldeoriola F . Neurophysiological correlate of clinical signs in Parkinson's disease. Clin Neurophysiol 2002; 113: 792–805.
Serranova T, Valls-Solé J, Muñoz E, Genís D, Jech R, Seeman P . Abnormal corticospinal tract modulation of the soleus H reflex in patients with pure spastic paraparesis. Neurosci Lett 2008; 437: 15–19.
Knikou M, Angeli CA, Ferreira CK, Harkema SJ . Soleus H-reflex modulation during body weight support treadmill walking in spinal cord intact and injured subjects. Exp Brain Res 2009; 193: 397–407.
American Spinal Injury Association. International Standards for Neurological Classification of Spinal Cord Injury, revised 2002. American Spinal Injury Association: Chicago, IL, 2002.
Dittuno PL, Dittuno Jr JF . Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord 2001; 39: 654–656.
Wolfe DL, Hayes KC, Potter PJ, Delaney GA . Conditioning lower limb H-reflexes by transcranial magnetic stimulation of motor cortex reveals preserved inervation in SCI patients. J Neurotrauma 1996; 13: 281–291.
Dimitrijevic MR, Kofler M, McKay WB, Sherwood AM, Van Der Linden C, Lissens MA . Early and late lower limb motor evoked potentials elicited by transcranial magnetic motor cortex stimulation. Electroencephalogr Clin Neurophysiol 1992; 85: 365–373.
Calancie B, Nordin W, Wallin U, Hagbarth K . Motor-unit responses in human wrist flexor and extensor muscles to transcranial cortical stimuli. J Neurophysiol 1987; 58: 1168–1185.
Holmgren H, Kadanza Z, Larsson L . Transcranial cortical stimulation. Late excitability changes in the soleus and tibial anterior motoneurone pools. Electroencephalogr Clin Neurophysiol 1992; 85: 374–381.
Acknowledgements
We thank Raquel López for help with statistical analyses and interpretation, and María Pérez for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Benito Penalva, J., Opisso, E., Medina, J. et al. H reflex modulation by transcranial magnetic stimulation in spinal cord injury subjects after gait training with electromechanical systems. Spinal Cord 48, 400–406 (2010). https://doi.org/10.1038/sc.2009.151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2009.151
Keywords
This article is cited by
-
Effects of postural and voluntary muscle contraction on modulation of the soleus H reflex by transcranial magnetic stimulation
Experimental Brain Research (2015)
-
The Walking Index for Spinal Cord Injury (WISCI/WISCI II): nature, metric properties, use and misuse
Spinal Cord (2013)
-
The effects of transcranial magnetic stimulation on vibratory-induced presynaptic inhibition of the soleus H reflex
Experimental Brain Research (2012)
-
Modulation of the soleus H reflex by electrical subcortical stimuli in humans
Experimental Brain Research (2011)