Abstract
Objective:
Idiopathic transverse myelitis (I-TM) is typically monophasic, while relapsing forms are usually referred to spinal cord-restricted neuromyelitis optica (NMO), atypical multiple sclerosis (MS), or myelitis during the course of infections and connectivitis. Our objective was to evaluate the frequency of recurrent I-TM; to clarify the nosology of these forms through comparison with NMO and post-infectious TM (P-TM).
Design:
Prospective cohort study on patients presenting with I-TM was carried out inpatients of Infectious and Neurologic Disease Clinics, Italy.
Methods:
Over an 8-year period, we recruited 13 patients with I-TM and 16 with P-TM. The patients were followed-up for at least 3 years with repeated brain and spinal cord magnetic resonance imaging (MRI) examinations, multimodal evoked potentials and serum screen for connectivitis. Relapses were defined on clinical and imaging criteria.
Results:
Four patients with I-TM (31%) had a relapsing course . They were all males with age >50, and severe at-onset disability. The final outcome was poor in three out of four patients. Serum NMO-immunoglobulin G was undetectable in all patients. Longitudinally extensive myelitis was not predictive of relapses. I-TM and P-TM shared clinical, cerebrospinal fluid (CSF) and MRI features, as well as a similar rate (54 vs 38%) of peripheral nervous system involvement (polyradiculoneuritis), and an identical rate of relapses (31% for both forms).
Conclusions:
Our series support the existence of relapsing I-TM as a disease entity that does not appear related to NMO, nor to MS, cannot be further specified and shares many features with P-TM. The likelihood of relapses was unpredictable based on clinical, CSF and MRI findings.
Similar content being viewed by others
Introduction
Recent criteria1 define transverse myelitis (TM) as the development, over a 3-week period, of sensory, motor or autonomic dysfunction related to spinal cord involvement, together with proof of spinal cord inflammation by cerebrospinal fluid (CSF) or magnetic resonance imaging (MRI). Depending on the results of an extensive infective and autoimmune screening, two main groups can be identified: idiopathic TM and ‘disease-associated’ TM. This distinction has prognostic purposes essentially. Disease associated forms may have a relapsing course, and differential diagnosis includes myelitis as the first manifestation of primarily central nervous system (CNS) diseases such as neuromyelitis optica (NMO) and multiple sclerosis (MS), as well as those related to direct infections and systemic autoimmune disorders. Conversely, I-TM is usually monophasic. Although recent studies on TM have applied this work-up,1 isolated cases or small series of relapsing I-TM are still reported,2, 3, 4 and their nosology remains controversial.3, 4, 5, 6 The final outcome is often characterized by severe disability,3 and treatment options for relapse prevention are not established.
Through a prospective, prolonged follow-up study, we previously evaluated the disease course of patients with post-infectious encephalomyelitis and myelitis, and could observe that 25% of patients had a relapsing course;7 relapses were confined to spinal cord involvement. We then observed the occurrence of relapsing TM among patients with hepatitis C virus (HCV) infection,8 and proposed that screening for HCV should be performed in each case of TM. In this study, we evaluate a series of I-TM, to assess the frequency of relapses; we then compare I-TM with a series of post-infectious TM (P-TM), monitored by our group as part of a previous study,7 to investigate differences between these forms. Last, we compare monophasic and relapsing myelitis, either idiopathic or post-infectious, to search for clinical, CSF or MRI predictors of a relapsing course.
Methods
Study design
We conducted a prospective cohort study on inpatients of Neurologic or Infectious Disease Clinics presenting with TM, over an 8-year period (between 1996 and December 2004). We excluded patients with TM associated with other CNS disorders, antecedent or direct infections, or systemic autoimmune disorders. The patients were followed-up for at least 3 years. Relapses were defined as: (1) sudden worsening of the neurological status after the achievement of a stabilization for at least 1 month, and, (2) new spinal cord lesion detectable by MRI, either at the same segment as the first episode (and thus visible as gadolinium enhancement), or at a different level (and thus identifiable as a new T2-hyperintense lesion, with or without enhancement, within the spinal cord).
Inclusion criteria
Transverse myelitis was diagnosed based on: (1) development of bilateral, not necessarily symmetrical, sensory-motor dysfunction with a clearly defined upper sensory level, with or without sphincter dysfunction; (2) blood–brain barrier damage, as expressed by increase in CSF/serum albumin ratio,9 or pleocytosis; (3) hyperintense-T2 lesions within the spinal cord, with or without enhancement. The neurological syndrome had to develop over less than 3 weeks, without antecedent infections or vaccinations during the previous 4 weeks.
Exclusion criteria
We excluded compressive, vascular and post-radiation myelopathies; MS (history of previous neurological signs or symptoms; demyelinating brain MRI lesions at inclusion or during follow-up; abnormal visual evoked potentials (VEPs)/brainstem auditory evoked potentials (BAEPs)); NMO (optic neuritis or abnormal VEPs); systemic autoimmune disorders and sarcoidosis; infectious diseases.
Screening for autoimmune disorders and infections has been described,7 and, briefly, it included serum search for antinuclear, anti-double-stranded DNA, anti-SSA/SSB, anti-neutrophil cytoplasmic, anticardiolipin and lupus anticoagulant antibodies; serum/CSF levels of angiotensin-converting enzyme; CSF culture; serum/CSF antibody titers of Epstein–Barr Virus (EBV), human immunodeficiency virus-1/-2, HCV, Borrelia burgdorferi, Mycoplasma pneumoniae; serum/CSF search by PCR for herpes simplex virus (HSV)-1/-2, enterovirus, EBV, cytomegalovirus, Varicella zoster virus, HHV-6, HHV-7, HHV-8, influenza A and B, HCV. Patients with serum hypereosinophilia were also excluded.
Assessments
Clinical impairment was assessed by the Scripps Neurological Rating Scale (SNRS) score10 at the time of the maximum neurological deficit and, after treatment, every 2 weeks, until the achievement of a stabilized neurological improvement.
Neuromyelitis optica-immunoglobulin G (IgG) antibodies were searched in all patients on collected serum samples, in accordance with a published protocol.11 In our laboratory, for the diagnosis of Devic's disease, NMO-IgG testing showed a sensitivity of 58%, and a specificity of 100%.
Serum C-reactive protein (CRP) was used to measure systemic inflammation. CSF examination included cell count, IgG and albumin content, glucose concentration, CSF/serum albumin ratio9 and IgG index. CSF and sera were tested for IgG oligoclonal bands (OBs) using agarose isoelectric focusing (pH 3.0–10.0) and affinity immunoblotting.
Spinal cord MRI was performed before the execution of rachicentesis, with a 1.5 T Philips Intera. Number, longitudinal length, localization of lesions and enhancement were recorded.
Neurophysiological assessment (VEPs, median/tibial somatosensory evoked potentials (SSEPs), BAEPs, nerve conduction studies) is described in a previous report,7 together with criteria for polyradiculoneuritis.12
Visual evoked potentials, SSEPs, brain and spinal cord MRI were repeated every 6 months during the 3-year follow-up, and promptly in relapses. In relapsing cases, CSF examination and serum and CSF screen for autoimmune disorders and infections was also repeated.
Treatment
The patients were treated with 6-methilprednisolone (6-MP) 500–1000 mg per day (until a maximum total dose of 6 g) as first choice, and with intravenous Ig (IVIg) when steroids were ineffective. Treatment ineffectiveness was defined as improvement of less than 30 SNSR points or final score <90 points.
Relapses were treated with 6-MP or IVIg, depending on which had been effective during the initial episode. Decisions on whether or not to start chronic immunosuppressive treatment in relapsing cases was taken on a individual basis, depending on the number of relapses, or the degree of residual disability after the first event (SNRS score > or <90). The choice among the agents commonly utilized for CNS autoimmune disorders was taken on an individual basis also.
Statistical analysis
We compared I-TM and P-TM, as well as monophasic and relapsing forms, considering the following quantitative variables: age, time to maximum neurological deficit, SNRS score at the onset and after treatment, CSF parameters, MRI number of segments involved by myelitis. The t-test or the Mann–Whitney test were adopted, depending on the distribution of the variables. The following categorical variables were considered: sex, indices of systemic inflammation, steroids/IVIg effectiveness, peripheral nerve involvement, pattern of spinal cord involvement on MRI (multifocal or single lesion; monosegmental or multisegmental lesions; site of lesion, both on axial and sagittal scans; presence of spinal cord swelling; enhancement; residual spinal cord atrophy), presence of relapses.
Patients
We considered for inclusion 21 patients with I-TM. At the end of the 3-year follow-up, two patients developed MS; one developed optic neuritis, and two developed bilateral delay on VEPs, and were classified as NMO (these three patients had negative NMO-IgG); three patients became positive to screening for anti-nuclear antibodies and anti-extractable nuclear antigens nucleolar antibodies and were also excluded. Thus, we identified 13 patients with I-TM.
During the same period, we identified 16 patients with P-TM. The most frequent antecedent event in P-TM was an aspecific, flu-like syndrome (8 of 16), followed by upper respiratory tract infections (5 of 16); two patients had pneumonia and one had gastroenteritis.
Clinical and instrumental features
Compared to P-TM, I-TM showed a trend to lower onset disability (F=2.69, P=0.08), negative CRP (F=4.4, P=0.067), lower CSF albumin (F=4.9, P=0.067), and lower CSF cells (F=4.9, P=0.060). I-TM and P-TM shared similar features as regard to age, sex, time to maximum deficit, steroid/IVIg effectiveness and residual disability. All patients presented with combined sensory, motor and sphincter dysfunction. The most common CSF abnormalities were increased albumin and IgG, and pleocytosis, with normal IgG index in all patients. Transitory CSF OBs were found in 2 of 13. All patients had negative NMO-IgG.
MRI showed a spectrum of abnormalities, with no significant differences between I-TM and P-TM as regard to the presence of multifocal lesions (23 vs 46%), single monosegmental lesions (23 vs 8%), longitudinally extensive TMs (LETMs; 54 vs 46%), these latter defined as lesions extending three or more contiguous vertebral segments on T2-weighted MRI images. Also, there were no significant differences between I-TM and P-TM as regard to median lesion extent, presence of mass effect (16 vs 43%), residual spinal cord atrophy (none vs 25%). On axial MRI, we could detect a mainly central, gray matter involvement, in both forms (66% of I-TM and in 73% of P-TM; Figure 1). We detected spinal cord enhancement in all but one patient, and lumbar root enhancement in 19% of I-TM and 25% of P-TM (Figure 2). The only statistically significant difference was the site of the lesions that were thoracic in 75% of I-TM, whereas P-TM showed a similar rate of thoracic vs cervical involvement (50%; χ2=6.17, P=0.046). In two patients with I-TM, a high cervical lesion extended to involve the low medulla.
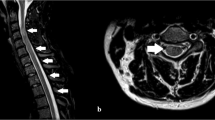
Sagittal T1-weighted images after gadolinium, of a patient with post-infectious transverse myelitis (TM) associated with polyradiculoneuritis. The image shows multiple multisegmental lesions, cervical and thoracic, with swelling of cervical medulla and conus. Contrast enhancement involves the cauda and conus also.
There was the same frequency of peripheral nervous system (PNS) involvement (7 of 13 patients, 54%, for I-TM, 5 of 16 patients, 38%, for P-TM), with mainly axonal damage in 4 of 7.
Results
Relapses
Of 13 patients, 4 with I-TM relapsed (31%): all were males above 50 years of age (Table 1). The total follow-up period was 38 months to 8 years. All patients had only one relapse, occurring 3–11 months after the initial episode, involving the same spinal segment of the first episode. Three of four patients had a severe final outcome. The relapses were treated with steroids (three patients) or with IVIg (one patient with steroid resistance). Methotrexate 10 mg i.m. weekly (one patient), i.v. cyclophosphamide 20 mg kg−1 per monthly (one patient), did not prevent progressive motor dysfunction.
Among P-TM, relapses occurred in 5 of 16 patients (31%) (Table 2), three women and two men, age range 24–71 years. The relapse occurred without any recognizable antecedent, with the exception of one patient relapsing 3 weeks after the administration of influenza vaccination. Time to first relapse was 5–14 months. Multiple relapses were observed in four of five patients. After one to three relapses, three patients began oral azathioprine 2 mg kg−1/daily (four patients), or cyclophosphamide (two patients) according to the above-specified doses, that did not prevent further relapses nor progressive worsening. Only in one patient, relapsed after the introduction of azathioprine, the administration of cyclophosphamide seemed effective in preventing further worsening: after a 5-year follow-up and four episodes of myelitis, she is now relapse free since 3 years, and still able to walk without support.
Comparison between monophasic and relapsing forms are shown in Tables 2 and 3 and in Figure 3.
Discussion
In our series, we found relapsing I-TM in 4 of 13 cases (31%), with the following features: (1) male gender; (2) age >50; (3) severe motor and sphincter dysfunction; (4) negative OBs, normal IgG index and negative NMO-IgG.
Consensus criteria for TM1 were not available when this prospective study was settled. Compared to these, our selection criteria additionally contemplate a 3-year follow-up, as well as the exclusion of patients with abnormal VEPs, and HCV infection. Indeed, recent reports classified as NMO are those recurrent myelitis with abnormal VEPs,5, 6 whereas others are identified as HCV infection in association with recurrent myelitis.8, 13
If we retrospectively apply to our patients, the criteria established by the TM Consortium Working Group, we can define our patients as definite TM based on clinical, CSF and spinal cord MRI features. About the recently proposed distinction between ‘partial’ and ‘complete’ myelitis,14 our patients, with prominent motor and sphincter dysfunction, and with a well-defined sensory level, fall in the group of ‘complete’, rather than partial, acute myelitis: this distinction aims to further separate forms with a likelihood of relapses from those that ,virtually, does not. Indeed, in our series, only two patients (2 of 21: 7%) who satisfied the inclusion criteria developed MS during the follow-up. Despite the stricter selection criteria1 and the inclusion of forms with severe motor impairment, still 30% of cases of ‘idiopathic’ TM were found to have a relapsing course, out of the context of MS, vasculitis, NMO, connectivitis and infections. Relapsing I-TM are reported in isolated reports and small series:2, 3, 4, 5 interpretations point at limited forms of NMO,5, 6 with possible subclinical optic nerve involvement, only detectable by VEPs or at a specific disease entity.3 Compared to NMO, our patients also showed severe neurological impairment, with prominent motor and sphincter dysfunction; CSF, pleocytosis; blood barrier damage; normal IgG index; absent or transitory OBs; on MRI, prominent gray matter involvement, with LETMs occurring in 54%. Two patients had lesions extending to the brainstem, a pattern described in an NMO series.6 However, we believe that recurrent I-TM in our patients was distinct from limited forms of NMO, for the following reasons: unlike NMO, our patients did not show optic nerve involvement, either clinical or subclinical; on MRI, we failed to detect T1-hypointense lesions and mass effect during the acute phase, or cavitations and areas of spinal cord atrophy on follow-up; in spite of the frequent occurrence of LETMs, as many as 46% patients had monosegmental lesions, possibly multifocal, that are unusual for NMO; ultimately, NMO-IgG were absent in all patients.
We rather found that I-TM shared many clinical, CSF and MRI features with P-TM. PNS involvement, a complication that is rarely observed myelitis other than post-infectious, was found in 54% of the I-TM, and in 30% of the ‘true’ P-TM. The features of PNS involvement, in the form of polyradiculoneuritis, distinct from Guillain–Barrè syndrome, were similar to those observed in P-TM:7 lack of albumin-cytologic dissociation; prominent axonal damage. Nerve roots involvement, the radiological counterpart of polyradiculoneuritis, was appreciable in 19% of cases (Figure 2). Compared to P-TM, we found only nonsignificant trends of differences: female prevalence; lower onset disability and higher disability after treatment; less obvious CSF signs of inflammation; less extensive lesions on sagittal scans, and cervical tract involvement less common. The two forms also shared the same rate of steroids ineffectiveness. As regard to relapses features, the only difference was a tendency to multiple relapses in P-TM, although we observed a single relapse among I-TM. In both forms, the relapse usually occurred at the same segment affected by the first episode (with only one exception in both groups), and without any recognizable antecedent infection (with the exception of influenza vaccination in one patient with P-TM).
Post-infectious TMs, also classically regarded as monophasic,1 are not contemplated by the TM-Working Group criteria. A few authors classify these forms as variants of acute disseminated encephalomyelitis (ADEM),15 but there is no general consensus about this classification. The possibility of relapses, usually confined to spinal cord involvement, is now contemplated as a complication of ADEM.16 Whether the presence or absence of an antecedent infection represents the only real difference between these two forms is not known. Comparing P-TM and I-TM, we found no real reason to separate these entities. The same rate of relapse was also found, reinforcing the similarities between the two forms. At least in studies concerning treatment modalities and prognosis, cases series on myelitis should include both post-infectious and idiopathic cases. In view of these similarities, we decided to compare monophasic and relapsing forms of both groups (P-TM and I-TM), and no clear differences emerged, with the limits represented by the low number of relapsing cases vs monophasic forms (9 vs 20). Relapses are almost invariably associated with a poor outcome, and relapse predictors, as well as effective treatment options for relapse prevention, remain to be established.
Disclosure/Conflict of interest
The authors state no conflict of interest.
References
Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology 2002; 59: 499–505.
Kim KK . Idiopathic recurrent transverse myelitis. Arch Neurol 2003; 60: 1290–1294.
Seifert T, Enzinger C, Ropele S, Storch MK, Strasser-Fuchs S, Fazekas F . Relapsing acute transverse myelitis: a specific entity. Eur J Neurol 2005; 12: 681–684.
Chan KH, Tsang KL, Fong GC, Ho SL, Cheung RT, Mak W . Idiopathic inflammatory demyelinating disorders after acute transverse myelitis. Eur J Neurol 2006; 13: 862–868.
Masuhr F, Busch M, Wetzel K, Harms L, Schielke E . Relapsing myelitis with pathological visual evoked potentials: a case of neuromyelitis optica? Eur J Neurol 2002; 9: 430–432.
Chan KH, Tsang KL, Fong GC, Cheung RT, Ho SL . Idiopathic severe recurrent transverse myelitis: a restricted variant of neuromyelitis optica. Clin Neurol Neurosurg 2005; 107: 132–135.
Marchioni E, Ravaglia S, Piccolo G, Furione M, Zardini E, Franciotta D et al. Postinfectious inflammatory disorders: subgroups based on prospective follow-up. Neurology 2005; 65: 1057–1065.
Aktipi KM, Ravaglia S, Ceroni M, Nemni R, Debiaggi M, Bastianello S et al. Severe recurrent myelitis in patients with hepatitis C virus infection. Neurology 2007; 68: 468–469.
Thompson EJ . The CSF Proteins: A Biochemical Approach. Elsevier: Amsterdam, 1988.
Koziol JA, Lucero A, Sipe JC, Romine JS, Beutler E . Responsiveness of the Scripps Neurologic Rating Scale during a multiple sclerosis clinical trial. Can J Neurol Sci 1999; 26: 283–289.
Lennon VA, Wingerchuk DM, Kryzer TJ, Pittock SJ, Lucchinetti CF, Fujihara K et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004; 364: 2106–2112.
Cornblath DR . Electrophysiology in Guillain–Barre syndrome. Ann Neurol 1990; 27 (Suppl): S17–S20.
Grewal AK, Lopes MB, Berg CL, Bennett AK, Alves VA, Trugman JM . Recurrent demyelinating myelitis associated with hepatitis C viral infection. J Neurol Sci 2004; 224: 101–106.
Scott TF . Nosology of idiopathic transverse myelitis syndromes. Acta Neurol Scand 2007; 115: 371–376.
al Deeb SM, Yaqub BA, Bruyn GW, Biary NM . Acute transverse myelitis. A localized form of postinfectious encephalomyelitis. Brain 1997; 120: 1115–1122.
Wingerchuk DM . The clinical course of acute disseminated encephalomyelitis. Neurol Res 2006; 28: 341–347.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravaglia, S., Bastianello, S., Franciotta, D. et al. NMO-IgG-negative relapsing myelitis. Spinal Cord 47, 531–537 (2009). https://doi.org/10.1038/sc.2008.157
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sc.2008.157
Keywords
This article is cited by
-
Acute transverse myelitis in demyelinating diseases among the Chinese
Journal of Neurology (2011)