Abstract
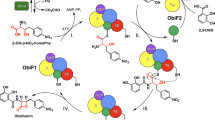
Naturally occurring β-lactone compounds are a class of strained four-membered heterocycles and can act as highly reactive electrophiles. Despite intensive studies for several decades, fewer than 30 β-lactones, many of which have high clinical potential, have been characterized from microorganisms. Here we report the discovery of a β-lactone compound, globilactone A through heterologous expression of a polyketide synthase/non-ribosomal peptide synthetase-like biosynthetic gene cluster (glo) in Streptomyces albus J1074. Biosynthetic pathway studies revealed that the polyketide synthase part can synthesize a polyunsaturated polyketide chain. While the downstream non-ribosomal peptide synthetase-like module, comprising condensation, FkbH, peptidyl carrier protein and thioester reductase domains (C–FkbH–PCP–R), incorporates a three-carbon pyruvate unit, and mediates formation of two carbon–carbon bonds between the polyketide and pyruvate to give a cyclopentane intermediate tethered on acyl carrier protein. A downstream esterase, GloD, plays a direct role in the β-lactone ring formation and releases the cyclopentane–β-lactone from the assembly line.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data supporting the findings of this work are available within the paper and its Supplementary Information files. The DNA sequence of gene cluster glo has been deposited in GenBank with the accession number OP620071 (https://www.ncbi.nlm.nih.gov/nuccore/OP620071.1/).The source data underlying Figs. 4a–d and 5c,d; Extended Data Figs. 2, 6a,b, 8a–c, 9a–c and 10a,b are provided as a Source Data file. Source data are provided with this paper.
References
Lowe, C. & Vederas, J. C. Naturally occuring β-lactones: occurence, synthesis and properties. A review. Org. Prep. Proced. Int. 27, 305–346 (1995).
Robinson, S. L., Christenson, J. K. & Wackett, L. P. Biosynthesis and chemical diversity of β-lactone natural products. Nat. Prod. Rep. 36, 458–475 (2019).
Nishimura, T. et al. JBIR-155, a specific class D β-lactamase inhibitor of microbial origin. Org. Lett. 23, 4415–4419 (2021).
Shi, Y. M. et al. Global analysis of biosynthetic gene clusters reveals conserved and unique natural products in entomopathogenic nematode-symbiotic bacteria. Nat. Chem. 14, 701–712 (2022).
De Pascale, G., Nazi, I., Harrison, P. H. & Wright, G. D. β-Lactone natural products and derivatives inactivate homoserine transacetylase, a target for antimicrobial agents. J. Antibiot. 64, 483–487 (2011).
Gulder, T. A. & Moore, B. S. Salinosporamide natural products: potent 20S proteasome inhibitors as promising cancer chemotherapeutics. Angew. Chem. Int. Ed. 49, 9346–9367 (2010).
Lall, M. S., Ramtohul, Y. K., James, M. N. G. & Vederas, J. C. Serine and threonine β-lactones: a new class of hepatitis A virus 3C cysteine proteinase inhibitors. J. Org. Chem. 67, 1536–1547 (2002).
Pemble, C. W. IV, Johnson, L. C., Kridel, S. J. & Lowther, W. T. Crystal structure of the thioesterase domain of human fatty acid synthase inhibited by Orlistat. Nat. Struct. Mol. Biol. 14, 704–709 (2007).
Derosa, G. & Maffioli, P. Anti-obesity drugs: a review about their effects and their safety. Expert Opin. Drug Saf. 11, 459–471 (2012).
Torgerson, J. S., Hauptman, J., Boldrin, M. N. & Sjöström, L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes Care 27, 155–161 (2004).
Roth, P. et al. A phase III trial of marizomib in combination with temozolomide-based radiochemotherapy versus temozolomide-based radiochemotherapy alone in patients with newly diagnosed glioblastoma. J. Clin. Oncol. 39, 2004 (2021).
Christenson, J. K. et al. β-Lactone synthetase found in the olefin biosynthesis pathway. Biochemistry 56, 348–351 (2017).
Schaffer, J. E., Reck, M. R., Prasad, N. K. & Wencewicz, T. A. β-Lactone formation during product release from a nonribosomal peptide synthetase. Nat. Chem. Biol. 13, 737–744 (2017).
Kreitler, D. F., Gemmell, E. M., Schaffer, J. E., Wencewicz, T. A. & Gulick, A. M. The structural basis of N-acyl-α-amino-β-lactone formation catalyzed by a nonribosomal peptide synthetase. Nat. Commun. 10, 3432–3445 (2019).
Feng, K.-N. et al. A hydrolase-catalyzed cyclization forms the fused bicyclic β-lactone in vibralactone. Angew. Chem. Int. Ed. 59, 7209–7213 (2020).
Bauman, K. D. et al. Enzymatic assembly of the salinosporamide γ-lactam–β-lactone anticancer warhead. Nat. Chem. Biol. 18, 538–546 (2022).
Shi, J. et al. Genome mining and biosynthesis of kitacinnamycins as a STING activator. Chem. Sci. 10, 4839–4846 (2019).
Shi, J. et al. Genome mining and enzymatic total biosynthesis of purincyclamide. Org. Lett. 21, 6825–6829 (2019).
Shi, J. et al. Discovery and biosynthesis of guanipiperazine from a NRPS-like pathway. Chem. Sci. 12, 2925–2930 (2021).
Blin, K. et al. antiSMASH 5.0: updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 47, W81–W87 (2019).
Chan, Y. A., Podevels, A. M., Kevany, B. M. & Thomas, M. G. Biosynthesis of polyketide synthase extender units. Nat. Prod. Rep. 26, 90–114 (2009).
Sun, Y., Hong, H., Gillies, F., Spencer, J. B. & Leadlay, P. F. Glyceryl-S-acyl carrier protein as an intermediate in the biosynthesis of tetronate antibiotics. ChemBioChem 9, 150–156 (2008).
Xu, Z. F. et al. Discovery and biosynthesis of bosamycins from Streptomyces sp. 120454. Chem. Sci. 11, 9237–9245 (2020).
Li, L. et al. The mildiomycin biosynthesis: initial steps for sequential generation of 5-hydroxymethylcytidine 5′-monophosphate and 5-hydroxymethylcytosine in Streptoverticillium rimofaciens ZJU5119. ChemBioChem 9, 1286–1294 (2008).
Liu, D. Z. et al. Vibralactone: a lipase inhibitor with an unusual fused β-lactone produced by cultures of the basidiomycete Boreostereum vibrans. Org. Lett. 8, 5749–5752 (2006).
Morinaka, A. et al. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J. Antimicrob. Chemother. 70, 2779–2786 (2015).
Kim, B. S., Cropp, T. A., Beck, B. J., Sherman, D. H. & Reynolds, K. A. Biochemical evidence for an editing role of thioesterase II in the biosynthesis of the polyketide pikromycin. J. Biol. Chem. 277, 48028–48034 (2002).
Geyer, K., Hartmann, S., Singh, R. R. & Erb, T. J. Multiple functions of the type II thioesterase associated with the phoslactomycin polyketide synthase. Biochemistry 61, 2662–2671 (2022).
Kotowska, M. & Pawlik, K. Roles of type II thioesterases and their application for secondary metabolite yield improvement. Appl. Microbiol. Biotechnol. 98, 7735–7746 (2014).
Romo, A. J. et al. The amipurimycin and miharamycin biosynthetic gene clusters: unraveling the origins of 2-aminopurinyl peptidyl nucleoside antibiotics. J. Am. Chem. Soc. 141, 14152–14159 (2019).
Etzbach, L., Plaza, A., Garcia, R., Baumann, S. & Müller, R. Cystomanamides: structure and biosynthetic pathway of a family of glycosylated lipopeptides from myxobacteria. Org. Lett. 16, 2414–2417 (2014).
Auerbach, D., Yan, F., Zhang, Y. & Müller, R. Characterization of an unusual glycerate esterification process in vioprolide biosynthesis. ACS Chem. Biol. 13, 3123–3130 (2018).
Agarwal, V. et al. Chemoenzymatic synthesis of acyl coenzyme a substrates enables in situ labeling of small molecules and proteins. Org. Lett. 17, 4452–4455 (2015).
Yi, D., Acharya, A., Gumbart, J. C., Gutekunst, W. R. & Agarwal, V. Gatekeeping ketosynthases dictate initiation of assembly line biosynthesis of pyrrolic polyketides. J. Am. Chem. Soc. 143, 7617–7622 (2021).
Calderone, C. T., Bumpus, S. B., Kelleher, N. L., Walsh, C. T. & Magarvey, N. A. A ketoreductase domain in the PksJ protein of the bacillaene assembly line carries out both α- and β-ketone reduction during chain growth. Proc. Natl Acad. Sci. USA 105, 12809–12814 (2008).
Scharf, D. H. et al. Epidithiol formation by an unprecedented twin carbon-sulfur lyase in the gliotoxin pathway. Angew. Chem. Int. Ed. 51, 10064–10068 (2012).
Agarwal, A., Kahyaoglu, C. & Hansen, D. B. Characterization of HmqF, a protein involved in the biosynthesis of unsaturated quinolones produced by Burkholderia thailandensis. Biochemistry 51, 1648–1657 (2012).
He, H. Y. et al. Quartromicin biosynthesis: two alternative polyketide chains produced by one polyketide synthase assembly line. Chem. Biol. 19, 1313–1323 (2012).
Süssmuth, R. D. & Mainz, A. Nonribosomal peptide synthesis—principles and prospects. Angew. Chem. Int. Ed. 56, 3770–3821 (2017).
Roche, E. D. & Walsh, C. T. Dissection of the EntF condensation domain boundary and active site residues in nonribosomal peptide synthesis. Biochemistry 42, 1334–1344 (2003).
Samel, S. A., Schoenafinger, G., Knappe, T. A., Marahiel, M. A. & Essen, L. O. Structural and functional insights into a peptide bond-forming bidomain from a nonribosomal peptide synthetase. Structure 15, 781–792 (2007).
Barajas, J. F. et al. Comprehensive structural and biochemical analysis of the terminal myxalamid reductase domain for the engineered production of primary alcohols. Chem. Biol. 22, 1018–1029 (2015).
Kopp, F., Mahlert, C., Grünewald, J. & Marahiel, M. A. Peptide macrocyclization: the reductase of the nostocyclopeptide synthetase triggers the self-assembly of a macrocyclic imine. J. Am. Chem. Soc. 128, 16478–16479 (2006).
He, H. Y., Yuan, H., Tang, M. C. & Tang, G. L. An unusual dehydratase acting on glycerate and a ketoreducatse stereoselectively reducing α-ketone in polyketide starter unit biosynthesis. Angew. Chem. Int. Ed. 53, 11315–11319 (2014).
Bretschneider, T. et al. Vinylogous chain branching catalysed by a dedicated polyketide synthase module. Nature 502, 124–128 (2013).
Geoghegan, K. F. et al. Spontaneous α-N-6-phosphogluconoylation of a ‘His tag’ in Escherichia coli: the cause of extra mass of 258 or 178 Da in fusion proteins. Anal. Biochem. 267, 169–184 (1999).
Acknowledgements
This research was financially supported by MOST (2022YFC2303100, 2022YFC2804100 and 2018YFA0902000), NSFC (81925033, 22193071, 22107048, 22077062, 81991522 and 81991524). We thank H. Zheng (China Pharmaceutical University) for his generous gift of expression plasmid NDM-1.
Author information
Authors and Affiliations
Contributions
Z.F.X., Y.L.Z. and S.T.B. carried out experiments. L.X., M.Y.X., J.S., B.Z. and R.X.T. assisted in NMR and MS data measurement and analysis. S.Q.Z., Z.R.X., D.Y. and B.S. contributed materials. Z.F.X. and H.M.G. wrote the paper. H.M.G. supervised the work. All authors discussed the results and analysed the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Synthesis thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Thomas West, in collaboration with the Nature Synthesis team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Enzymatic mechanisms of known β-lactone ring biosynthesis.
cis-3-octyl-4-nonyloxetan-2-one (a), obafluorin (b), vibralactone (c), salinosporamide A (d).
Extended Data Fig. 2 Inhibitory activitiy against the class A β-lactamase of 1.
An IC50 value against class A β-lactamase of 1 was 15.05 μM.
Extended Data Fig. 3 Biochemical characterization of GloE.
a Reaction scheme for the GloE activity assay. b HPLC analysis of the enzymatic assays of GloE with 4. c HPLC analysis of the enzymatic assays of GloE with 1.
Extended Data Fig. 4 Labeling patterns of globilactone C (3) and metabolic intermediates after feeding 13C-labeled glucoses.
Heavy bars denote contiguous 13C labels of connected atoms, dots denote single 13C labels.
Extended Data Fig. 5 Chemoenzymatic synthesis of 8-S-ACP (GloB) and 12-S-GloC GloD.
Reaction scheme depicting chemoenzymatic synthesis and subsequent acylation assay to generate 8-S-ACP (GloB), 8-S-ACP-C-FkbH (GloB) (a) and 13-S-GloC-GloD (b).
Extended Data Fig. 6 In vitro reconstitution of 10.
a LC-MS analysis of the one-pot enzymatic total biosynthesis of 10. i) boiled holo-GloC-GloD, 8-S-ACP-C-FkbH (GloB), 1,3-BPG, NADPH and N-acetyl-L-cysteine; ii) holo-GloC-GloD, 8-S-ACP-C-FkbH (GloB), 1,3-BPG and N-acetyl-L-cysteine; iii) holo-GloC-GloD, 8-S-ACP-C-FkbH (GloB), 1,3-BPG, NADPH and N-acetyl-L-cysteine. b high-resolution MS (HRMS) data of 10.
Extended Data Fig. 7 Chemoenzymatic synthesis of 11-S-ACP (GloB) and 11-S-ACP-C-FkbH (GloB).
Reaction scheme depicting chemoenzymatic synthesis and subsequent acylation assay to generate 11-S-ACP-C-FkbH (GloB).
Extended Data Fig. 8 Mass spectrometric analysis of production of acylated ACP.
a holo-ACP (GloB). b ACP-tethered linear substrate (8-S-ACP) from pantetheine-activated (8c) via in vitro CoA biosynthesis and Sfp-mediated loading. c holo-GloC-GloD, holo-ACP-C-FkbH (GloB), 1,3-BPG, NADPH and 8-S-ACP. Additional peaks marked by asterisks represent adventitious gluconylation (+178 Da) of N-terminal His6-tag during expression of the recombinant protein in E. coli.
Extended Data Fig. 9 MS2 data localized the active site to the ACP, where the serine (S) is the site of 4′-Ppant and mass shifts were confirmed through measurement of the 4′-Ppant elimination product.
a holo-ACP (GloB). b ACP-tethered linear substrate (8-S-ACP) from pantetheine-activated (8c) via in vitro CoA biosynthesis and Sfp-mediated loading. c holo-GloC-GloD, holo-ACP-C-FkbH (GloB), 1,3-BPG, NADPH and 8-S-ACP.
Extended Data Fig. 10 LC-MS analysis of ACP-C-FkbH (GloB) and GloD active site mutagenesis.
a LC-MS analysis of the one-pot enzymatic total biosynthesis of 3. b Mass spectrometric analysis of production of acylated ACP (12). Additional peaks marked by asterisks represent adventitious gluconylation (+178 Da) of N-terminal His6-tag during expression of the recombinant protein in E. coli.
Supplementary information
Source data
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig./Table 2
Statistical source data.
Source Data Extended Data Fig./Table 6
Statistical source data.
Source Data Extended Data Fig./Table 8
Statistical source data.
Source Data Extended Data Fig./Table 9
Statistical source data.
Source Data Extended Data Fig./Table 10
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, Z.F., Zhou, Y.L., Bo, S.T. et al. Discovery and biosynthetic pathway analysis of cyclopentane–β-lactone globilactone A. Nat. Synth 3, 99–110 (2024). https://doi.org/10.1038/s44160-023-00414-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s44160-023-00414-3