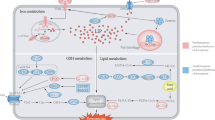
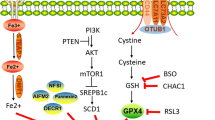
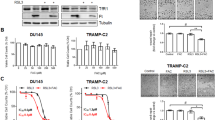
Abstract
Ferroptosis is a distinct form of regulated cell death that is predominantly driven by the build-up of intracellular iron and lipid peroxides. Ferroptosis suppression is widely accepted to contribute to the pathogenesis of several tumours including prostate cancer. Results from some studies reported that prostate cancer cells can be highly susceptible to ferroptosis inducers, providing potential for an interesting new avenue of therapeutic intervention for advanced prostate cancer. In this Perspective, we describe novel molecular underpinnings and metabolic drivers of ferroptosis, analyse the functions and mechanisms of ferroptosis in tumours, and highlight prostate cancer-specific susceptibilities to ferroptosis by connecting ferroptosis pathways to the distinctive metabolic reprogramming of prostate cancer cells. Leveraging these novel mechanistic insights could provide innovative therapeutic opportunities in which ferroptosis induction augments the efficacy of currently available prostate cancer treatment regimens, pending the elimination of major bottlenecks for the clinical translation of these treatment combinations, such as the development of clinical-grade inhibitors of the anti-ferroptotic enzymes as well as non-invasive biomarkers of ferroptosis. These biomarkers could be exploited for diagnostic imaging and treatment decision-making.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel, R. L., Miller, K. D., Wagle, N. S. & Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 73, 17–48 (2023).
Moreira, D. M. et al. Predicting time from metastasis to overall survival in castration-resistant prostate cancer: results from SEARCH. Clin. Genitourin. Cancer 15, 60–66. e62 (2017).
Chen, X., Comish, P. B., Tang, D. & Kang, R. Characteristics and biomarkers of ferroptosis. Front. Cell Dev. Biol. 9, 637162 (2021).
Yang, W. S. et al. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc. Natl Acad. Sci. USA 113, E4966–E4975 (2016).
Lin, Z. et al. The lipid flippase SLC47A1 blocks metabolic vulnerability to ferroptosis. Nat. Commun. 13, 7965 (2022).
Catala, A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem. Phys. Lipids 157, 1–11 (2009).
Gao, M. et al. Role of mitochondria in ferroptosis. Mol. Cell 73, 354–363 e353 (2019).
Park, M. W. et al. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 41, 101947 (2021).
Yan, B. et al. Membrane damage during ferroptosis is caused by oxidation of phospholipids catalyzed by the oxidoreductases POR and CYB5R1. Mol. Cell 81, 355–369 e310 (2021).
Zhang, S. et al. Double-edge sword roles of iron in driving energy production versus instigating ferroptosis. Cell Death Dis. 13, 40 (2022).
von Krusenstiern, A. N. et al. Identification of essential sites of lipid peroxidation in ferroptosis. Nat. Chem. Biol. 19, 719–730 (2023).
Yang, W. S. & Stockwell, B. R. Ferroptosis: death by lipid peroxidation. Trends Cell Biol. 26, 165–176 (2016).
Conrad, M. & Friedmann Angeli, J. P. Glutathione peroxidase 4 (Gpx4) and ferroptosis: what’s so special about it? Mol. Cell Oncol. 2, e995047 (2015).
Lu, S. C. Glutathione synthesis. Biochim. Biophys. Acta 1830, 3143–3153 (2013).
Du, Y. & Guo, Z. Recent progress in ferroptosis: inducers and inhibitors. Cell Death Discov. 8, 501 (2022).
Li, Q. et al. Understanding sorafenib-induced ferroptosis and resistance mechanisms: implications for cancer therapy. Eur. J. Pharmacol. 955, 175913 (2023).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Yan, H. F. et al. Ferroptosis: mechanisms and links with diseases. Signal. Transduct. Target. Ther. 6, 49 (2021).
Mao, C. et al. DHODH-mediated ferroptosis defence is a targetable vulnerability in cancer. Nature 593, 586–590 (2021).
Cirilli, I. et al. Role of coenzyme Q10 in health and disease: an update on the last 10 years (2010–2020). Antioxidants 10, 1325 (2021).
Bentinger, M., Tekle, M. & Dallner, G. Coenzyme Q — biosynthesis and functions. Biochem. Biophys. Res. Commun. 396, 74–79 (2010).
Kraft, V. A. N. et al. GTP cyclohydrolase 1/tetrahydrobiopterin counteract ferroptosis through lipid remodeling. ACS Cent. Sci. 6, 41–53 (2020).
Lei, G., Zhuang, L. & Gan, B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer 22, 381–396 (2022).
Dixon, S. J. et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. Elife 3, e02523 (2014).
Jeong, S. D. et al. Enhanced immunogenic cell death by apoptosis/ferroptosis hybrid pathway potentiates PD-L1 blockade cancer immunotherapy. ACS Biomater. Sci. Eng. 8, 5188–5198 (2022).
Lei, G. et al. The role of ferroptosis in ionizing radiation-induced cell death and tumor suppression. Cell Res. 30, 146–162 (2020).
Viswanathan, V. S. et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 547, 453–457 (2017).
Tousignant, K. D. et al. Therapy-induced lipid uptake and remodeling underpin ferroptosis hypersensitivity in prostate cancer. Cancer Metab. 8, 11 (2020).
Ghoochani, A. et al. Ferroptosis inducers are a novel therapeutic approach for advanced prostate cancer. Cancer Res. 81, 1583–1594 (2021).
Yang, Y. et al. Ferroptosis inducer erastin downregulates androgen receptor and its splice variants in castration-resistant prostate cancer. Oncol. Rep. 45, 25 (2021).
Mathur, D. et al. PTEN regulates glutamine flux to pyrimidine synthesis and sensitivity to dihydroorotate dehydrogenase inhibition. Cancer Discov. 7, 380–390 (2017).
Lv, Z. et al. Identifying a ferroptosis-related gene signature for predicting biochemical recurrence of prostate cancer. Front. Cell Dev. Biol. 9, 666025 (2021).
Mao, C., Liu, X., Yan, Y., Olszewski, K. & Gan, B. Reply to: DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E19–E23 (2023).
Mishima, E. et al. DHODH inhibitors sensitize to ferroptosis by FSP1 inhibition. Nature 619, E9–E18 (2023).
Yoo, S. E. et al. Gpx4 ablation in adult mice results in a lethal phenotype accompanied by neuronal loss in brain. Free. Radic. Biol. Med. 52, 1820–1827 (2012).
Mei, J., Webb, S., Zhang, B. & Shu, H. B. The p53-inducible apoptotic protein AMID is not required for normal development and tumor suppression. Oncogene 25, 849–856 (2006).
Doll, S. et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat. Chem. Biol. 13, 91–98 (2017).
Rodriguez, R., Schreiber, S. L. & Conrad, M. Persister cancer cells: iron addiction and vulnerability to ferroptosis. Mol. Cell 82, 728–740 (2022).
Scheinberg, T., Mak, B., Butler, L., Selth, L. & Horvath, L. G. Targeting lipid metabolism in metastatic prostate cancer. Ther. Adv. Med. Oncol. 15, 17588359231152839 (2023).
Mah, C. Y., Nassar, Z. D., Swinnen, J. V. & Butler, L. M. Lipogenic effects of androgen signaling in normal and malignant prostate. Asian J. Urol. 7, 258–270 (2020).
Poulose, N. et al. Genetics of lipid metabolism in prostate cancer. Nat. Genet. 50, 169–171 (2018).
Scaglia, N., Frontini-Lopez, Y. R. & Zadra, G. Prostate cancer progression: as a matter of fats. Front. Oncol. 11, 719865 (2021).
Castelli, S., De Falco, P., Ciccarone, F., Desideri, E. & Ciriolo, M. R. Lipid catabolism and ROS in cancer: a bidirectional liaison. Cancers 13, 5484 (2021).
Iglesias-Gato, D. et al. The proteome of primary prostate cancer. Eur. Urol. 69, 942–952 (2016).
Magtanong, L. et al. Exogenous monounsaturated fatty acids promote a ferroptosis-resistant cell state. Cell Chem. Biol. 26, 420–432 e429 (2019).
Das, U. N. Saturated fatty acids, MUFAs and PUFAs regulate ferroptosis. Cell Chem. Biol. 26, 309–311 (2019).
Yuan, H., Li, X., Zhang, X., Kang, R. & Tang, D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem. Biophys. Res. Commun. 478, 1338–1343 (2016).
Yang, Y. et al. ACSL3 and ACSL4, distinct roles in ferroptosis and cancers. Cancers 14, 5896 (2022).
Doll, S. & Conrad, M. Iron and ferroptosis: a still ill-defined liaison. IUBMB Life 69, 423–434 (2017).
Ma, Y. et al. Long-chain Acyl-CoA synthetase 4-mediated fatty acid metabolism sustains androgen receptor pathway-independent prostate cancer. Mol. Cancer Res. 19, 124–135 (2021).
Marques, R. B. et al. Modulation of androgen receptor signaling in hormonal therapy-resistant prostate cancer cell lines. PLoS ONE 6, e23144 (2011).
Wu, X. et al. ACSL4 promotes prostate cancer growth, invasion and hormonal resistance. Oncotarget 6, 44849–44863 (2015).
Wang, M. E. et al. RB1-deficient prostate tumor growth and metastasis are vulnerable to ferroptosis induction via the E2F/ACSL4 axis. J. Clin. Invest. 133, e166647 (2023).
Orlando, U. D. et al. Acyl-CoA synthetase-4 is implicated in drug resistance in breast cancer cell lines involving the regulation of energy-dependent transporter expression. Biochem. Pharmacol. 159, 52–63 (2019).
Sanchez-Martinez, R., Cruz-Gil, S., Garcia-Alvarez, M. S., Reglero, G. & Ramirez de Molina, A. Complementary ACSL isoforms contribute to a non-Warburg advantageous energetic status characterizing invasive colon cancer cells. Sci. Rep. 7, 11143 (2017).
Wu, X. et al. Long chain fatty Acyl-CoA synthetase 4 is a biomarker for and mediator of hormone resistance in human breast cancer. PLoS ONE 8, e77060 (2013).
Xia, H. et al. Simultaneous silencing of ACSL4 and induction of GADD45B in hepatocellular carcinoma cells amplifies the synergistic therapeutic effect of aspirin and sorafenib. Cell Death Discov. 3, 17058 (2017).
Mashima, T., Seimiya, H. & Tsuruo, T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br. J. Cancer 100, 1369–1372 (2009).
Sena, L. A. & Denmeade, S. R. Fatty acid synthesis in prostate cancer: vulnerability or epiphenomenon? Cancer Res. 81, 4385–4393 (2021).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Swinnen, J. V., Brusselmans, K. & Verhoeven, G. Increased lipogenesis in cancer cells: new players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 9, 358–365 (2006).
Swinnen, J. V., Esquenet, M., Goossens, K., Heyns, W. & Verhoeven, G. Androgens stimulate fatty acid synthase in the human prostate cancer cell line LNCaP. Cancer Res. 57, 1086–1090 (1997).
Heemers, H. V., Verhoeven, G. & Swinnen, J. V. Androgen activation of the sterol regulatory element-binding protein pathway: current insights. Mol. Endocrinol. 20, 2265–2277 (2006).
Butler, L. M., Centenera, M. M. & Swinnen, J. V. Androgen control of lipid metabolism in prostate cancer: novel insights and future applications. Endocr. Relat. Cancer 23, R219–227, (2016).
Han, L. Q., Gao, T. Y., Yang, G. Y. & Loor, J. J. Overexpression of SREBF chaperone (SCAP) enhances nuclear SREBP1 translocation to upregulate fatty acid synthase (FASN) gene expression in bovine mammary epithelial cells. J. Dairy. Sci. 101, 6523–6531 (2018).
Rossi, S. et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol. Cancer Res. 1, 707–715 (2003).
Shah, U. S. et al. Fatty acid synthase gene overexpression and copy number gain in prostate adenocarcinoma. Hum. Pathol. 37, 401–409 (2006).
Epstein, J. I., Carmichael, M. & Partin, A. W. OA-519 (fatty acid synthase) as an independent predictor of pathologic state in adenocarcinoma of the prostate. Urology 45, 81–86 (1995).
Song, X. et al. PDK4 dictates metabolic resistance to ferroptosis by suppressing pyruvate oxidation and fatty acid synthesis. Cell Rep. 34, 108767 (2021).
Li, C. et al. LKB1-AMPK axis negatively regulates ferroptosis by inhibiting fatty acid synthesis. Signal. Transduct. Target. Ther. 5, 187 (2020).
Lee, H. et al. Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234 (2020).
Bartolacci, C. et al. Targeting de novo lipogenesis and the Lands cycle induces ferroptosis in KRAS-mutant lung cancer. Nat. Commun. 13, 4327 (2022).
Hardie, D. G., Ross, F. A. & Hawley, S. A. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 13, 251–262 (2012).
Park, H. U. et al. AMP-activated protein kinase promotes human prostate cancer cell growth and survival. Mol. Cancer Ther. 8, 733–741 (2009).
Tennakoon, J. B. et al. Androgens regulate prostate cancer cell growth via an AMPK-PGC-1ɑ-mediated metabolic switch. Oncogene 33, 5251–5261 (2014).
Lin, C. et al. Inhibition of CAMKK2 impairs autophagy and castration-resistant prostate cancer via suppression of AMPK-ULK1 signaling. Oncogene 40, 1690–1705 (2021).
Khan, A. S. & Frigo, D. E. A spatiotemporal hypothesis for the regulation, role, and targeting of AMPK in prostate cancer. Nat. Rev. Urol. 14, 164–180 (2017).
Frigo, D. E. et al. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 71, 528–537 (2011).
Enoch, H. G., Catala, A. & Strittmatter, P. Mechanism of rat liver microsomal stearyl-CoA desaturase. Studies of the substrate specificity, enzyme-substrate interactions, and the function of lipid. J. Biol. Chem. 251, 5095–5103 (1976).
Amezaga, J. et al. Altered red blood cell membrane fatty acid profile in cancer patients. Nutrients 10, 1853 (2018).
Chavarro, J. E. et al. Blood levels of saturated and monounsaturated fatty acids as markers of de novo lipogenesis and risk of prostate cancer. Am. J. Epidemiol. 178, 1246–1255 (2013).
Fritz, V. et al. Abrogation of de novo lipogenesis by stearoyl-CoA desaturase 1 inhibition interferes with oncogenic signaling and blocks prostate cancer progression in mice. Mol. Cancer Ther. 9, 1740–1754 (2010).
Yi, J., Zhu, J., Wu, J., Thompson, C. B. & Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl Acad. Sci. USA 117, 31189–31197 (2020).
Tesfay, L. et al. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 79, 5355–5366 (2019).
Kagan, V. E. et al. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat. Chem. Biol. 13, 81–90 (2017).
D’Angelo, S., Motti, M. L. & Meccariello, R. ω-3 and ω-6 polyunsaturated fatty acids, obesity and cancer. Nutrients 12, 2751 (2020).
Lee, J. M., Lee, H., Kang, S. & Park, W. J. Fatty acid desaturases, polyunsaturated fatty acid regulation, and biotechnological advances. Nutrients 8, 23 (2016).
Lee, J. Y. et al. Polyunsaturated fatty acid biosynthesis pathway determines ferroptosis sensitivity in gastric cancer. Proc. Natl Acad. Sci. USA 117, 32433–32442 (2020).
Yamane, D. et al. FADS2-dependent fatty acid desaturation dictates cellular sensitivity to ferroptosis and permissiveness for hepatitis C virus replication. Cell Chem. Biol. 29, 799–810 e794 (2022).
Xu, H. et al. ELOVL5-mediated long chain fatty acid elongation contributes to enzalutamide resistance of prostate cancer. Cancers 13, 3957 (2021).
Centenera, M. M. et al. ELOVL5 is a critical and targetable fatty acid elongase in prostate cancer. Cancer Res. 81, 1704–1718 (2021).
Schlaepfer, I. R. & Joshi, M. CPT1A-mediated fat oxidation, mechanisms, and therapeutic potential. Endocrinology 161, bqz046 (2020).
Flaig, T. W. et al. Lipid catabolism inhibition sensitizes prostate cancer cells to antiandrogen blockade. Oncotarget 8, 56051–56065 (2017).
Joshi, M. et al. CPT1A supports castration-resistant prostate cancer in androgen-deprived conditions. Cells 8, 115 (2019).
Nassar, Z. D. et al. Human DECR1 is an androgen-repressed survival factor that regulates PUFA oxidation to protect prostate tumor cells from ferroptosis. Elife 9, e54166 (2020).
Gao, M., Monian, P., Quadri, N., Ramasamy, R. & Jiang, X. Glutaminolysis and transferrin regulate ferroptosis. Mol. Cell 59, 298–308 (2015).
Philpott, C. C. et al. Iron-tracking strategies: chaperones capture iron in the cytosolic labile iron pool. Front. Mol. Biosci. 10, 1127690 (2023).
Yanatori, I., Richardson, D. R., Imada, K. & Kishi, F. Iron export through the transporter ferroportin 1 is modulated by the iron chaperone PCBP2. J. Biol. Chem. 291, 17303–17318 (2016).
Donovan, A. et al. The iron exporter ferroportin/Slc40a1 is essential for iron homeostasis. Cell Metab. 1, 191–200 (2005).
Nemeth, E. & Ganz, T. The role of hepcidin in iron metabolism. Acta Haematol. 122, 78–86 (2009).
Hubert, N. & Hentze, M. W. Previously uncharacterized isoforms of divalent metal transporter (DMT)-1: implications for regulation and cellular function. Proc. Natl Acad. Sci. USA 99, 12345–12350 (2002).
Dai, E., Meng, L., Kang, R., Wang, X. & Tang, D. ESCRT-III-dependent membrane repair blocks ferroptosis. Biochem. Biophys. Res. Commun. 522, 415–421 (2020).
Vela, D. Iron metabolism in prostate cancer; from basic science to new therapeutic strategies. Front. Oncol. 8, 547 (2018).
Torti, S. V., Manz, D. H., Paul, B. T., Blanchette-Farra, N. & Torti, F. M. Iron and cancer. Annu. Rev. Nutr. 38, 97–125 (2018).
Brown, R. A. M. et al. Altered iron metabolism and impact in cancer biology, metastasis, and immunology. Front. Oncol. 10, 476 (2020).
Deng, Z., Manz, D. H., Torti, S. V. & Torti, F. M. Iron-responsive element-binding protein 2 plays an essential role in regulating prostate cancer cell growth. Oncotarget 8, 82231–82243 (2017).
Keer, H. N. et al. Elevated transferrin receptor content in human prostate cancer cell lines assessed in vitro and in vivo. J. Urol. 143, 381–385 (1990).
Kuvibidila, S., Gauthier, T., Warrier, R. P. & Rayford, W. Increased levels of serum transferrin receptor and serum transferrin receptor/log ferritin ratios in men with prostate cancer and the implications for body-iron stores. J. Lab. Clin. Med. 144, 176–182 (2004).
Xue, D., Zhou, C. X., Shi, Y. B., Lu, H. & He, X. Z. Decreased expression of ferroportin in prostate cancer. Oncol. Lett. 10, 913–916 (2015).
Burnell, S. E. A. et al. STEAP2 knockdown reduces the invasive potential of prostate cancer cells. Sci. Rep. 8, 6252 (2018).
Hou, W. et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy 12, 1425–1428 (2016).
Winterbourn, C. C. Toxicity of iron and hydrogen peroxide: the Fenton reaction. Toxicol. Lett. 82-83, 969–974 (1995).
Dhur, A., Galan, P. & Hercberg, S. Effects of different degrees of iron deficiency on cytochrome P450 complex and pentose phosphate pathway dehydrogenases in the rat. J. Nutr. 119, 40–47 (1989).
Pistorius, E. K. & Axelrod, B. Iron, an essential component of lipoxygenase. J. Biol. Chem. 249, 3183–3186 (1974).
Yauger, Y. J. et al. Iron accentuated reactive oxygen species release by NADPH oxidase in activated microglia contributes to oxidative stress in vitro. J. Neuroinflammation 16, 41 (2019).
Bordini, J. et al. Induction of iron excess restricts malignant plasma cells expansion and potentiates bortezomib effect in models of multiple myeloma. Leukemia 31, 967–970 (2017).
Campanella, A. et al. Iron increases the susceptibility of multiple myeloma cells to bortezomib. Haematologica 98, 971–979 (2013).
Bordini, J. et al. Iron induces cell death and strengthens the efficacy of antiandrogen therapy in prostate cancer models. Clin. Cancer Res. 26, 6387–6398 (2020).
Maccarinelli, F. et al. Iron supplementation enhances RSL3-induced ferroptosis to treat naive and prevent castration-resistant prostate cancer. Cell Death Discov. 9, 81 (2023).
Liu, Y. et al. Liposomes embedded with PEGylated iron oxide nanoparticles enable ferroptosis and combination therapy in cancer. Natl Sci. Rev. 10, nwac167 (2023).
Fernandez-Acosta, R. et al. Novel iron oxide nanoparticles induce ferroptosis in a panel of cancer cell lines. Molecules 27, 3970 (2022).
Bonvin, D. et al. Tuning properties of iron oxide nanoparticles in aqueous synthesis without ligands to improve MRI relaxivity and SAR. Nanomaterials 7, 225 (2017).
Hajikarimi, Z., Khoei, S., Khoee, S. & Mahdavi, S. R. Evaluation of the cytotoxic effects of PLGA coated iron oxide nanoparticles as a carrier of 5- fluorouracil and mega-voltage X-ray radiation in DU145 prostate cancer cell line. IEEE Trans. Nanobiosci. 13, 403–408 (2014).
Kader, A. et al. Iron oxide nanoparticles for visualization of prostate cancer in MRI. Cancers 14, 2909 (2022).
Cluntun, A. A., Lukey, M. J., Cerione, R. A. & Locasale, J. W. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer 3, 169–180 (2017).
Gong, T. et al. Glutamine metabolism in cancers: targeting the oxidative homeostasis. Front. Oncol. 12, 994672 (2022).
Wang, Q. et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J. Pathol. 236, 278–289 (2015).
Wang, Q. et al. Androgen receptor and nutrient signaling pathways coordinate the demand for increased amino acid transport during prostate cancer progression. Cancer Res. 71, 7525–7536 (2011).
Cardoso, H. J. et al. Glutaminolysis is a metabolic route essential for survival and growth of prostate cancer cells and a target of 5ɑ-dihydrotestosterone regulation. Cell Oncol. 44, 385–403 (2021).
Serpa, J. Cysteine as a carbon source, a hot spot in cancer cells survival. Front. Oncol. 10, 947 (2020).
Poltorack, C. D. & Dixon, S. J. Understanding the role of cysteine in ferroptosis: progress & paradoxes. FEBS J. 289, 374–385 (2022).
Yang, J., Dai, X., Xu, H., Tang, Q. & Bi, F. Regulation of ferroptosis by amino acid metabolism in cancer. Int. J. Biol. Sci. 18, 1695–1705 (2022).
Zhong, W. et al. Extracellular redox state shift: a novel approach to target prostate cancer invasion. Free. Radic. Biol. Med. 117, 99–109 (2018).
Doxsee, D. W. et al. Sulfasalazine-induced cystine starvation: potential use for prostate cancer therapy. Prostate 67, 162–171 (2007).
Cramer, S. L. et al. Systemic depletion of L-cyst(e)ine with cyst(e)inase increases reactive oxygen species and suppresses tumor growth. Nat. Med. 23, 120–127 (2017).
Mandigo, A. C. et al. RB/E2F1 as a master regulator of cancer cell metabolism in advanced disease. Cancer Discov. 11, 2334–2353 (2021).
Nikfar, S., Rahimi, R., Rezaie, A. & Abdollahi, M. A meta-analysis of the efficacy of sulfasalazine in comparison with 5-aminosalicylates in the induction of improvement and maintenance of remission in patients with ulcerative colitis. Dig. Dis. Sci. 54, 1157–1170 (2009).
Rains, C. P., Noble, S. & Faulds, D. Sulfasalazine. A review of its pharmacological properties and therapeutic efficacy in the treatment of rheumatoid arthritis. Drugs 50, 137–156 (1995).
Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 10, 194–204 (2010).
Ogura, T., Tanaka, Y., Tamaki, H. & Harada, M. Docetaxel induces Bcl-2- and pro-apoptotic caspase-independent death of human prostate cancer DU145 cells. Int. J. Oncol. 48, 2330–2338 (2016).
Darshan, M. S. et al. Taxane-induced blockade to nuclear accumulation of the androgen receptor predicts clinical responses in metastatic prostate cancer. Cancer Res. 71, 6019–6029 (2011).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Galsky, M. D., Dritselis, A., Kirkpatrick, P. & Oh, W. K. Cabazitaxel. Nat. Rev. Drug. Discov. 9, 677–678 (2010).
Chen, X. et al. Ferroptosis induction improves the sensitivity of docetaxel in prostate cancer. Oxid. Med. Cell. Longev. 2022, 1–16 (2022).
Jiang, X. et al. TFAP2C-mediated lncRNA PCAT1 inhibits ferroptosis in docetaxel-resistant prostate cancer through c-Myc/miR-25-3p/SLC7A11 signaling. Front. Oncol. 12, 862015 (2022).
He, S. et al. ChaC glutathione specific γ-glutamylcyclotransferase 1 inhibits cell viability and increases the sensitivity of prostate cancer cells to docetaxel by inducing endoplasmic reticulum stress and ferroptosis. Exp. Ther. Med. 22, 997 (2021).
Ogawa, T. et al. CHAC1 overexpression in human gastric parietal cells with Helicobacter pylori infection in the secretory canaliculi. Helicobacter 24, e12598 (2019).
Takeda, D. Y. et al. A somatically acquired enhancer of the androgen receptor is a noncoding driver in advanced prostate cancer. Cell 174, 422–432 e413 (2018).
Schweizer, M. T. & Yu, E. Y. Persistent androgen receptor addiction in castration-resistant prostate cancer. J. Hematol. Oncol. 8, 128 (2015).
Abida, W. et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc. Natl Acad. Sci. USA 116, 11428–11436 (2019).
Watson, P. A., Arora, V. K. & Sawyers, C. L. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat. Rev. Cancer 15, 701–711 (2015).
Cattrini, C. et al. Apalutamide, darolutamide and enzalutamide for nonmetastatic castration-resistant prostate cancer (nmCRPC): a critical review. Cancers 14, 1492 (2022).
Desai, K., McManus, J. M. & Sharifi, N. Hormonal therapy for prostate cancer. Endocr. Rev. 42, 354–373 (2021).
Clarke, N. W. et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 1, EVIDoa2200043 (2022).
Chi, K. N. et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J. Clin. Oncol. 41, 3339–3351 (2023).
Hong, T. et al. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 42, 101928 (2021).
Sugiura, M. et al. Identification of AR-V7 downstream genes commonly targeted by AR/AR-V7 and specifically targeted by AR-V7 in castration resistant prostate cancer. Transl. Oncol. 14, 100915 (2021).
Yang, F. et al. Ferroptosis heterogeneity in triple-negative breast cancer reveals an innovative immunotherapy combination strategy. Cell Metab. 35, 84–100.e8 (2023).
Sun, R. et al. Androgen receptor variants confer castration resistance in prostate cancer by counteracting antiandrogen-induced ferroptosis. Cancer Res. 83, 3192–3204 (2023).
Liang, D. et al. Ferroptosis surveillance independent of GPX4 and differentially regulated by sex hormones. Cell 186, 2748–2764 e2722 (2023).
Ma, D. et al. Crystal structure of a membrane-bound O-acyltransferase. Nature 562, 286–290 (2018).
Masumoto, N. et al. Membrane bound O-acyltransferases and their inhibitors. Biochem. Soc. Trans. 43, 246–252 (2015).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Runcie, K. D. & Dallos, M. C. Prostate cancer immunotherapy — finally in from the cold? Curr. Oncol. Rep. 23, 88 (2021).
Graff, J. N. et al. Phase II study of ipilimumab in men with metastatic prostate cancer with an incomplete response to androgen deprivation therapy. Front. Oncol. 10, 1381 (2020).
Fizazi, K. et al. Final analysis of the ipilimumab versus placebo following radiotherapy phase III trial in postdocetaxel metastatic castration-resistant prostate cancer identifies an excess of long-term survivors. Eur. Urol. 78, 822–830 (2020).
Cabel, L. et al. Long-term complete remission with Ipilimumab in metastatic castrate-resistant prostate cancer: case report of two patients. J. Immunother. Cancer 5, 31 (2017).
Sharma, P. et al. Nivolumab plus ipilimumab for metastatic castration-resistant prostate cancer: preliminary analysis of patients in the checkmate 650 trial. Cancer Cell 38, 489–499 e483 (2020).
Fizazi, K. et al. Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial. J. Immunother. Cancer 10, e004761 (2022).
Fizazi, K. et al. Nivolumab plus docetaxel in patients with chemotherapy-naive metastatic castration-resistant prostate cancer: results from the phase II CheckMate 9KD trial. Eur. J. Cancer 160, 61–71 (2022).
Petrylak, D. P. et al. KEYNOTE-921: phase III study of pembrolizumab plus docetaxel for metastatic castration-resistant prostate cancer. Future Oncol. 17, 3291–3299 (2021).
Antonarakis, E. S. et al. Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J. Clin. Oncol. 38, 395–405 (2020).
Petrylak, D. P. et al. Safety and clinical activity of atezolizumab in patients with metastatic castration-resistant prostate cancer: a phase I study. Clin. Cancer Res. 27, 3360–3369 (2021).
Powles, T. et al. Atezolizumab with enzalutamide versus enzalutamide alone in metastatic castration-resistant prostate cancer: a randomized phase 3 trial. Nat. Med. 28, 144–153 (2022).
Rodriguez-Vida, A. et al. Safety and efficacy of avelumab plus carboplatin in patients with metastatic castration-resistant prostate cancer in an open-label Phase Ib study. Br. J. Cancer 128, 21–29 (2023).
Kwan, E. M. et al. Avelumab combined with stereotactic ablative body radiotherapy in metastatic castration-resistant prostate cancer: the phase 2 ICE-PAC clinical trial. Eur. Urol. 81, 253–262 (2022).
Karzai, F. et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J. Immunother. Cancer 6, 141 (2018).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Shore, N. D. et al. CD8+ T cells impact rising PSA in biochemically relapsed cancer patients using immunotherapy targeting tumor-associated antigens. Mol. Ther. 28, 1238–1250 (2020).
Kyriakopoulos, C. E. et al. Multicenter phase I trial of a DNA vaccine encoding the androgen receptor ligand-binding domain (pTVG-AR, MVI-118) in patients with metastatic prostate cancer. Clin. Cancer Res. 26, 5162–5171 (2020).
Singh, P., Pal, S. K., Alex, A. & Agarwal, N. Development of PROSTVAC immunotherapy in prostate cancer. Future Oncol. 11, 2137–2148 (2015).
Gulley, J. L. et al. Phase III trial of PROSTVAC in asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. J. Clin. Oncol. 37, 1051–1061 (2019).
van den Eertwegh, A. J. et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factor-transduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 13, 509–517 (2012).
Small, E. J. et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clin. Cancer Res. 13, 3883–3891 (2007).
Higano, C. S. et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer 113, 975–984 (2008).
Tschernia, N. P., Norberg, S. M. & Gulley, J. L. CAR T cells reach clinical milestone in prostate cancer. Nat. Med. 28, 635–636 (2022).
Perera, M. P. J. et al. Chimeric antigen receptor T-cell therapy in metastatic castrate-resistant prostate cancer. Cancers 14, 503 (2022).
Narayan, V. et al. PSMA-targeting TGFβ-insensitive armored CAR T cells in metastatic castration-resistant prostate cancer: a phase 1 trial. Nat. Med. 28, 724–734 (2022).
Yunger, S. et al. Tumor-infiltrating lymphocytes from human prostate tumors reveal anti-tumor reactivity and potential for adoptive cell therapy. Oncoimmunology 8, e1672494 (2019).
Karbach, J. et al. Tumor-infiltrating lymphocytes mediate complete and durable remission in a patient with NY-ESO-1 expressing prostate cancer. J. Immunother. Cancer 11, e005847 (2023).
Kamat, N. V., Yu, E. Y. & Lee, J. K. BiTE-ing into prostate cancer with bispecific T-cell engagers. Clin. Cancer Res. 27, 2675–2677 (2021).
Hummel, H. D. et al. Pasotuxizumab, a BiTE® immune therapy for castration-resistant prostate cancer: phase I, dose-escalation study findings. Immunotherapy 13, 125–141 (2021).
Wang, W. et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 569, 270–274 (2019).
Liao, P. et al. CD8+ T cells and fatty acids orchestrate tumor ferroptosis and immunity via ACSL4. Cancer Cell 40, 365–378.e6 (2022).
Jiang, Z. et al. TYRO3 induces anti-PD-1/PD-L1 therapy resistance by limiting innate immunity and tumoral ferroptosis. J. Clin. Invest. 131, e139434 (2021).
Efimova, I. et al. Vaccination with early ferroptotic cancer cells induces efficient antitumor immunity. J. Immunother. Cancer 8, e001369 (2020).
Tang, D., Kepp, O. & Kroemer, G. Ferroptosis becomes immunogenic: implications for anticancer treatments. Oncoimmunology 10, 1862949 (2020).
Wen, Q., Liu, J., Kang, R., Zhou, B. & Tang, D. The release and activity of HMGB1 in ferroptosis. Biochem. Biophys. Res. Commun. 510, 278–283 (2019).
Yu, B., Choi, B., Li, W. & Kim, D. H. Magnetic field boosted ferroptosis-like cell death and responsive MRI using hybrid vesicles for cancer immunotherapy. Nat. Commun. 11, 3637 (2020).
Wu, Z. et al. The landscape of immune cells infiltrating in prostate cancer. Front. Oncol. 10, 517637 (2020).
Martin, A. M. et al. Paucity of PD-L1 expression in prostate cancer: innate and adaptive immune resistance. Prostate Cancer Prostatic Dis. 18, 325–332 (2015).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
Ma, X. et al. CD36-mediated ferroptosis dampens intratumoral CD8+ T cell effector function and impairs their antitumor ability. Cell Metab. 33, 1001–1012.e5 (2021).
Zhou, X. et al. Abrogation of HnRNP L enhances anti-PD-1 therapy efficacy via diminishing PD-L1 and promoting CD8+ T cell-mediated ferroptosis in castration-resistant prostate cancer. Acta Pharm. Sin. B 12, 692–707 (2022).
Sindhu, K. K., Nehlsen, A. D. & Stock, R. G. Radium-223 for metastatic castrate-resistant prostate cancer. Pract. Radiat. Oncol. 12, 312–316 (2022).
Parker, C. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 369, 213–223 (2013).
Violet, J. et al. Dosimetry of 177Lu-PSMA-617 in metastatic castration-resistant prostate cancer: correlations between pretherapeutic imaging and whole-body tumor dosimetry with treatment outcomes. J. Nucl. Med. 60, 517–523 (2019).
Sun, M., Niaz, M. O., Nelson, A., Skafida, M. & Niaz, M. J. Review of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer. Cureus 12, e8921 (2020).
Schuchardt, C. et al. Prostate-specific membrane antigen radioligand therapy using 177Lu-PSMA I&T and 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: comparison of safety, biodistribution, and dosimetry. J. Nucl. Med. 63, 1199–1207 (2022).
Kratochwil, C. et al. 225Ac-PSMA-617 for PSMA-targeted ɑ-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 57, 1941–1944 (2016).
Hammer, S. et al. Preclinical efficacy of a PSMA-targeted thorium-227 conjugate (PSMA-TTC), a targeted ɑ therapy for prostate cancer. Clin. Cancer Res. 26, 1985–1996 (2020).
Kiess, A. P. et al. 2S)-2-(3-(1-Carboxy-5-(4-211At-Astatobenzamido)Pentyl)Ureido)-pentanedioic acid for PSMA-targeted ɑ-particle radiopharmaceutical therapy. J. Nucl. Med. 57, 1569–1575 (2016).
Ye, L. F. et al. Radiation-induced lipid peroxidation triggers ferroptosis and synergizes with ferroptosis inducers. ACS Chem. Biol. 15, 469–484 (2020).
Lang, X. et al. Radiotherapy and immunotherapy promote tumoral lipid oxidation and ferroptosis via synergistic repression of SLC7A11. Cancer Discov. 9, 1673–1685 (2019).
Lowe, S. W., Schmitt, E. M., Smith, S. W., Osborne, B. A. & Jacks, T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature 362, 847–849 (1993).
Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51, 6304–6311 (1991).
Jiang, L. et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520, 57–62 (2015).
Wang, Y. et al. Epigenetic regulation of ferroptosis by H2B monoubiquitination and p53. EMBO Rep. 20, e47563 (2019).
Shen, D. et al. PARPi treatment enhances radiotherapy-induced ferroptosis and antitumor immune responses via the cGAS signaling pathway in colorectal cancer. Cancer Lett. 550, 215919 (2022).
Lei, G., Mao, C., Yan, Y., Zhuang, L. & Gan, B. Ferroptosis, radiotherapy, and combination therapeutic strategies. Protein Cell 12, 836–857 (2021).
Beretta, G. L. & Zaffaroni, N. Radiotherapy-induced ferroptosis for cancer treatment. Front. Mol. Biosci. 10, 1216733 (2023).
Upadhyayula, P. S. et al. Dietary restriction of cysteine and methionine sensitizes gliomas to ferroptosis and induces alterations in energetic metabolism. Nat. Commun. 14, 1187 (2023).
Xue, Y. et al. Intermittent dietary methionine deprivation facilitates tumoral ferroptosis and synergizes with checkpoint blockade. Nat. Commun. 14, 4758 (2023).
Dierge, E. et al. Peroxidation of n-3 and n-6 polyunsaturated fatty acids in the acidic tumor environment leads to ferroptosis-mediated anticancer effects. Cell Metab. 33, 1701–1715 e1705 (2021).
Ferrer, M. et al. Ketogenic diet promotes tumor ferroptosis but induces relative corticosterone deficiency that accelerates cachexia. Cell Metab. 35, 1147–1162 e1147 (2023).
Pissios, P. et al. Methionine and choline regulate the metabolic phenotype of a ketogenic diet. Mol. Metab. 2, 306–313 (2013).
Eaton, J. K. et al. Selective covalent targeting of GPX4 using masked nitrile-oxide electrophiles. Nat. Chem. Biol. 16, 497–506 (2020).
Eaton, J. K., Ruberto, R. A., Kramm, A., Viswanathan, V. S. & Schreiber, S. L. Diacylfuroxans are masked nitrile oxides that inhibit GPX4 covalently. J. Am. Chem. Soc. 141, 20407–20415 (2019).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Koppula, P. et al. A targetable CoQ-FSP1 axis drives ferroptosis- and radiation-resistance in KEAP1 inactive lung cancers. Nat. Commun. 13, 2206 (2022).
Doll, S. et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature 575, 693–698 (2019).
Xavier da Silva, T. N., Schulte, C., Alves, A. N., Maric, H. M. & Friedmann Angeli, J. P. Molecular characterization of AIFM2/FSP1 inhibition by iFSP1-like molecules. Cell Death Dis. 14, 281 (2023).
Hendricks, J. M. et al. Identification of structurally diverse FSP1 inhibitors that sensitize cancer cells to ferroptosis. Cell Chem. Biol. 30, 1090–1103 e1097 (2023).
Yoshioka, H. et al. Identification of a small molecule that enhances ferroptosis via inhibition of ferroptosis suppressor protein 1 (FSP1). ACS Chem. Biol. 17, 483–491 (2022).
Antoszczak, M. & Huczynski, A. Salinomycin and its derivatives — a new class of multiple-targeted “magic bullets”. Eur. J. Med. Chem. 176, 208–227 (2019).
Miyazaki, Y., Shibuya, M., Sugawara, H., Kawaguchi, O. & Hirsoe, C. Salinomycin, a new polyether antibiotic. J. Antibiot. 27, 814–821 (1974).
Zhou, S. et al. Salinomycin: a novel anti-cancer agent with known anti-coccidial activities. Curr. Med. Chem. 20, 4095–4101 (2013).
Mai, T. T. et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 9, 1025–1033 (2017).
Mohammad, A. et al. Abstract 3107: HSB-1216, a novel agent for the treatment of chemotherapy-resistant ES-SCLC. Cancer Res. 81, 3107–3107 (2021).
Kharbanda, S. et al. Novel iron-mediated cell death (Ferroptosis) inducer, HSB-1216, suppress acute myeloid leukemia growth. Eur. J. Cancer https://doi.org/10.1016/s0959-8049(22)00936-4 (2022).
Li, J. et al. Ferroptosis: past, present and future. Cell Death Dis. 11, 88 (2020).
Kloditz, K. & Fadeel, B. Three cell deaths and a funeral: macrophage clearance of cells undergoing distinct modes of cell death. Cell Death Discov. 5, 65 (2019).
Zou, Y. & Schreiber, S. L. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem. Biol. 27, 463–471 (2020).
Li, Z. et al. In vivo tracking cystine/glutamate antiporter-mediated cysteine/cystine pool under ferroptosis. Anal. Chim. Acta 1125, 66–75 (2020).
Li, Z. Imaging of hydrogen peroxide (H(2)O(2)) during the ferroptosis process in living cancer cells with a practical fluorescence probe. Talanta 212, 120804 (2020).
Conrad, M. & Pratt, D. A. The chemical basis of ferroptosis. Nat. Chem. Biol. 15, 1137–1147 (2019).
Zeng, F. et al. Ferroptosis detection: from approaches to applications. Angew. Chem. Int. Ed. 62, e202300379 (2023).
Li, J., Kang, R. & Tang, D. Monitoring autophagy-dependent ferroptosis. Methods Cell Biol. 165, 163–176 (2021).
Wang, F. et al. PALP: a rapid imaging technique for stratifying ferroptosis sensitivity in normal and tumor tissues in situ. Cell Chem. Biol. 29, 157–170 e156 (2022).
Wang, F., Naowarojna, N. & Zou, Y. Stratifying ferroptosis sensitivity in cells and mouse tissues by photochemical activation of lipid peroxidation and fluorescent imaging. Star. Protoc. 3, 101189 (2022).
Drummen, G. P., van Liebergen, L. C., Op den Kamp, J. A. & Post, J. A. C11-BODIPY(581/591), an oxidation-sensitive fluorescent lipid peroxidation probe: (micro)spectroscopic characterization and validation of methodology. Free. Radic. Biol. Med. 33, 473–490 (2002).
Hirayama, T., Okuda, K. & Nagasawa, H. A highly selective turn-on fluorescent probe for iron(II) to visualize labile iron in living cells. Chem. Sci. 4, 1250–1256, (2013).
Kapralov, A. A. et al. Redox lipid reprogramming commands susceptibility of macrophages and microglia to ferroptotic death. Nat. Chem. Biol. 16, 278–290 (2020).
Weigand, I. et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 11, 192 (2020).
Zhao, N. et al. Ferronostics: measuring tumoral ferrous iron with PET to predict sensitivity to iron-targeted cancer therapies. J. Nucl. Med. 62, 949–955 (2021).
Phyo, A. P. et al. Antimalarial activity of artefenomel (OZ439), a novel synthetic antimalarial endoperoxide, in patients with Plasmodium falciparum and Plasmodium vivax malaria: an open-label phase 2 trial. Lancet Infect. Dis. 16, 61–69 (2016).
Feng, H. et al. Transferrin receptor is a specific ferroptosis marker. Cell Rep. 30, 3411–3423 e3417 (2020).
Shibata, Y., Yasui, H., Higashikawa, K. & Kuge, Y. Transferrin-based radiolabeled probe predicts the sensitivity of human renal cancer cell lines to ferroptosis inducer erastin. Biochem. Biophys. Rep. 26, 100957 (2021).
McCormick, P. N. et al. Assessment of tumor redox status through (S)-4-(3-[18F]fluoropropyl)-L-glutamic acid PET imaging of system xc− activity. Cancer Res. 79, 853–863 (2019).
Hoehne, A. et al. [18F]FSPG-PET reveals increased cystine/glutamate antiporter (xc-) activity in a mouse model of multiple sclerosis. J. Neuroinflammation 15, 55 (2018).
Park, S. Y. et al. Clinical evaluation of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate (18F-FSPG) for PET/CT imaging in patients with newly diagnosed and recurrent prostate cancer. Clin. Cancer Res. 26, 5380–5387 (2020).
Park, S. Y. et al. Initial evaluation of (4S)-4-(3-[18F]fluoropropyl)-L-glutamate (FSPG) PET/CT imaging in patients with head and neck cancer, colorectal cancer, or non-Hodgkin lymphoma. EJNMMI Res. 10, 100 (2020).
Zeng, F. et al. Ferroptosis MRI for early detection of anticancer drug-induced acute cardiac/kidney injuries. Sci. Adv. 9, eadd8539 (2023).
Zhang, C. et al. Fe-based theranostic agents respond to the tumor microenvironment for MRI-guided ferroptosis-/apoptosis-inducing anticancer therapy. ACS Biomater. Sci. Eng. 8, 2610–2623 (2022).
Zhu, L. et al. Efficient magnetic nanocatalyst-induced chemo- and ferroptosis synergistic cancer therapy in combination with T1-T2 dual-mode magnetic resonance imaging through doxorubicin delivery. ACS Appl. Mater. Interfaces 14, 3621–3632 (2022).
Chen, Q. et al. Iron-based nanoparticles for MR imaging-guided ferroptosis in combination with photodynamic therapy to enhance cancer treatment. Nanoscale 13, 4855–4870 (2021).
Zheng, J. & Conrad, M. The metabolic underpinnings of ferroptosis. Cell Metab. 32, 920–937 (2020).
Bayir, H. et al. Achieving life through death: redox biology of lipid peroxidation in ferroptosis. Cell Chem. Biol. 27, 387–408 (2020).
Friedmann Angeli, J. P., Krysko, D. V. & Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 19, 405–414 (2019).
Gu, Y. et al. Targeting ferroptosis: paving new roads for drug design and discovery. Eur. J. Med. Chem. 247, 115015 (2023).
Wang, D. et al. Regulatory pathways and drugs associated with ferroptosis in tumors. Cell Death Dis. 13, 544 (2022).
Jin, J. et al. Machine learning classifies ferroptosis and apoptosis cell death modalities with TfR1 immunostaining. ACS Chem. Biol. 17, 654–660 (2022).
Shibata, Y., Yasui, H., Higashikawa, K., Miyamoto, N. & Kuge, Y. Erastin, a ferroptosis-inducing agent, sensitized cancer cells to X-ray irradiation via glutathione starvation in vitro and in vivo. PLoS ONE 14, e0225931 (2019).
Liu, W. et al. Ferroptosis inducer improves the efficacy of oncolytic virus-mediated cancer immunotherapy. Biomedicines 10, 1425 (2022).
Lu, Z. et al. Combined anti-cancer effects of platycodin D and sorafenib on androgen-independent and PTEN-deficient prostate cancer. Front. Oncol. 11, 648985 (2021).
Oh, S. J., Erb, H. H., Hobisch, A., Santer, F. R. & Culig, Z. Sorafenib decreases proliferation and induces apoptosis of prostate cancer cells by inhibition of the androgen receptor and Akt signaling pathways. Endocr. Relat. Cancer 19, 305–319 (2012).
Roh, J. L., Kim, E. H., Jang, H. & Shin, D. Aspirin plus sorafenib potentiates cisplatin cytotoxicity in resistant head and neck cancer cells through xCT inhibition. Free. Radic. Biol. Med. 104, 1–9 (2017).
Cobler, L., Zhang, H., Suri, P., Park, C. & Timmerman, L. A. xCT inhibition sensitizes tumors to gamma-radiation via glutathione reduction. Oncotarget 9, 32280–32297 (2018).
Nagane, M. et al. Sulfasalazine, an inhibitor of the cystine-glutamate antiporter, reduces DNA damage repair and enhances radiosensitivity in murine B16F10 melanoma. PLoS ONE 13, e0195151 (2018).
Shamaa, M. M. Sulfasalazine synergistically enhances the inhibitory effects of imatinib against hepatocellular carcinoma (HCC) cells by targeting NFκB, BCR/ABL, and PI3K/AKT signaling pathway-related proteins. FEBS Open. Bio 11, 588–597 (2021).
Chen, C. et al. Flubendazole plays an important anti-tumor role in different types of cancers. Int. J. Mol. Sci. 23, 519 (2022).
Zhou, X. et al. Flubendazole, FDA-approved anthelmintic, elicits valid antitumor effects by targeting P53 and promoting ferroptosis in castration-resistant prostate cancer. Pharmacol. Res. 164, 105305 (2021).
Li, M. et al. RSL3 enhances the antitumor effect of cisplatin on prostate cancer cells via causing glycolysis dysfunction. Biochem. Pharmacol. 192, 114741 (2021).
Wang, J. et al. Inhibition of phosphoglycerate dehydrogenase induces ferroptosis and overcomes enzalutamide resistance in castration-resistant prostate cancer cells. Drug Resist. Updat. 70, 100985 (2023).
Zhao, R. et al. ATF6ɑ promotes prostate cancer progression by enhancing PLA2G4A-mediated arachidonic acid metabolism and protecting tumor cells against ferroptosis. Prostate 82, 617–629 (2022).
Wang, H. et al. Discovery of ML210-Based glutathione peroxidase 4 (GPX4) degrader inducing ferroptosis of human cancer cells. Eur. J. Med. Chem. 254, 115343 (2023).
Eling, N., Reuter, L., Hazin, J., Hamacher-Brady, A. & Brady, N. R. Identification of artesunate as a specific activator of ferroptosis in pancreatic cancer cells. Oncoscience 2, 517–532 (2015).
Luo, J. et al. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat. Oncol. 9, 84 (2014).
Yin, X. et al. Artesunate suppresses the proliferation and development of estrogen receptor-alpha-positive endometrial cancer in HAND2-dependent pathway. Front. Cell Dev. Biol. 8, 606969 (2020).
Zhang, Z. Y. et al. [Artesunate combined with vinorelbine plus cisplatin in treatment of advanced non-small cell lung cancer: a randomized controlled trial]. Zhong Xi Yi Jie He Xue Bao 6, 134–138 (2008).
Sun, Y. et al. Fin56-induced ferroptosis is supported by autophagy-mediated GPX4 degradation and functions synergistically with mTOR inhibition to kill bladder cancer cells. Cell Death Dis. 12, 1028 (2021).
Khalil, R. et al. Withaferin A increases the effectiveness of immune checkpoint blocker for the treatment of non-small cell lung cancer. Cancers 15, 3089 (2023).
Kim, S. H. et al. RNA-seq reveals novel mechanistic targets of withaferin A in prostate cancer cells. Carcinogenesis 41, 778–789 (2020).
Kyakulaga, A. H., Aqil, F., Munagala, R. & Gupta, R. C. Synergistic combinations of paclitaxel and withaferin A against human non-small cell lung cancer cells. Oncotarget 11, 1399–1416 (2020).
Nishikawa, Y. et al. Withaferin A induces cell death selectively in androgen-independent prostate cancer cells but not in normal fibroblast cells. PLoS ONE 10, e0134137 (2015).
Yang, E. S., Choi, M. J., Kim, J. H., Choi, K. S. & Kwon, T. K. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol. Vitr. 25, 1803–1810 (2011).
Kristensen, G. B., Baekelandt, M., Vergote, I. B. & Trope, C. A phase II study of carboplatin and hexamethylmelamine as induction chemotherapy in advanced epithelial ovarian carcinoma. Eur. J. Cancer 31A, 1778–1780 (1995).
Malik, I. A. Altretamine is an effective palliative therapy of patients with recurrent epithelial ovarian cancer. Jpn. J. Clin. Oncol. 31, 69–73 (2001).
Woo, J. H. et al. Elucidating compound mechanism of action by network perturbation analysis. Cell 162, 441–451 (2015).
Wang, L., Chen, X. & Yan, C. Ferroptosis: an emerging therapeutic opportunity for cancer. Genes. Dis. 9, 334–346 (2022).
Qin, Z. et al. Design and synthesis of isothiocyanate-containing hybrid androgen receptor (AR) antagonist to downregulate AR and induce ferroptosis in GSH-deficient prostate cancer cells. Chem. Biol. Drug. Des. 97, 1059–1078 (2021).
Li, Q. et al. The effects of buthionine sulfoximine on the proliferation and apoptosis of biliary tract cancer cells induced by cisplatin and gemcitabine. Oncol. Lett. 11, 474–480 (2016).
Bump, E. A. & Brown, J. M. Role of glutathione in the radiation response of mammalian cells in vitro and in vivo. Pharmacol. Ther. 47, 117–136 (1990).
Guo, J. et al. Ferroptosis: a novel anti-tumor action for cisplatin. Cancer Res. Treat. 50, 445–460 (2018).
Efferth, T. Cancer combination therapies with artemisinin-type drugs. Biochem. Pharmacol. 139, 56–70 (2017).
Sundar, S. N., Marconett, C. N., Doan, V. B., Willoughby, J. A. Sr & Firestone, G. L. Artemisinin selectively decreases functional levels of estrogen receptor-ɑ and ablates estrogen-induced proliferation in human breast cancer cells. Carcinogenesis 29, 2252–2258 (2008).
Wang, T. et al. Combination treatment with artemisinin and oxaliplatin inhibits tumorigenesis in esophageal cancer EC109 cell through Wnt/β-catenin signaling pathway. Thorac. Cancer 11, 2316–2324 (2020).
Willoughby, J. A. Sr. et al. Artemisinin blocks prostate cancer growth and cell cycle progression by disrupting Sp1 interactions with the cyclin-dependent kinase-4 (CDK4) promoter and inhibiting CDK4 gene expression. J. Biol. Chem. 284, 2203–2213 (2009).
Dai, X. et al. Dihydroartemisinin: a potential natural anticancer drug. Int. J. Biol. Sci. 17, 603–622 (2021).
Han, W. et al. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. Biomaterials 280, 121315 (2022).
Li, Q. et al. Dihydroartemisinin as a sensitizing agent in cancer therapies. Onco Targets Ther. 14, 2563–2573 (2021).
Lin, R. et al. Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett. 381, 165–175 (2016).
Abrams, R. P., Carroll, W. L. & Woerpel, K. A. Five-membered ring peroxide selectively initiates ferroptosis in cancer cells. ACS Chem. Biol. 11, 1305–1312 (2016).
Gaschler, M. M. et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat. Chem. Biol. 14, 507–515 (2018).
Saha, A. et al. Cysteine depletion sensitizes prostate cancer cells to agents that enhance DNA damage and to immune checkpoint inhibition. J. Exp. Clin. Cancer Res. 42, 119 (2023).
Badgley, M. A. et al. Cysteine depletion induces pancreatic tumor ferroptosis in mice. Science 368, 85–89 (2020).
Acknowledgements
We are thankful to D. Chalaire, Editing Services, Research Medical Library, The UT MD Anderson Cancer Center for helping us with the editing of the manuscript. This work was supported by grants from the Department of Defense (W81XWH-19-1-0410, W81XWH-22-1-0686 and HT9425-23-1-0424 (D.E.F.)), the National Institutes of Health (R01CA184208 (D.E.F.)), an UT MD Anderson Cancer Center Quantitative Imaging Analysis Core (QIAC) Pilot Grant (D.E.F.), and a grant from the Mike Slive Foundation for Prostate Cancer Research (D.E.F.). This work was also supported by Marilyn and Frederick R. Lummis, Jr., M.D., Fellowship in Biomedical Sciences (P.H.A.C.).
Author information
Authors and Affiliations
Contributions
D.E.F., P.H.A.C., A.D., F.E.L., S.S. and E.S. researched data for the article. D.E.F., P.H.A.C., A.D., P.K.B. and E.S. contributed substantially to discussion of the content. P.H.A.C., A.D., F.E.L., S.S. and E.S. wrote the article. D.E.F., P.H.A.C., A.D., P.K.B. and E.S. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
D.E.F. has received research funding from GTx, Inc., and has a familial relationship with Biocity Biopharmaceuticals, Hummingbird Bioscience, Bellicum Pharmaceuticals, Maia Biotechnology, Alms Therapeutics, Hinova Pharmaceuticals and Barricade Therapeutics. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, P.H.A., Dominic, A., Lujan, F.E. et al. Unlocking ferroptosis in prostate cancer — the road to novel therapies and imaging markers. Nat Rev Urol (2024). https://doi.org/10.1038/s41585-024-00869-9
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-024-00869-9