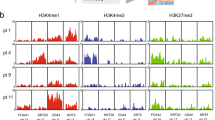
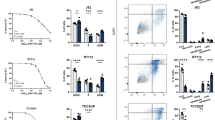
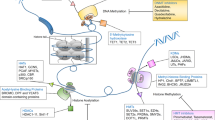
Abstract
Bladder cancer in the most advanced, muscle-invasive stage is lethal, and very limited therapeutic advances have been reported for decades. To date, cisplatin-based chemotherapy remains the first-line therapy for advanced bladder cancer. Late-line options have historically been limited. In the past few years, next-generation sequencing technology has enabled chromatin remodelling gene mutations to be characterized, showing that these alterations are more frequent in urothelial bladder carcinoma than in other cancer types. Histone modifiers have functional roles in tumour progression by modulating the expression of tumour suppressors and oncogenes and, therefore, have been considered as novel drug targets for cancer therapy. The roles of epigenetic reprogramming through histone modifications have been increasingly studied in bladder cancer, and the therapeutic efficacy of targeting those histone modifiers genetically or chemically is being assessed in preclinical studies. Results from preclinical studies in bladder cancer encouraged the investigation of some of these drugs in clinical trials, which yield mixed results. Further understanding of how alterations of histone modification mechanistically contribute to bladder cancer progression, drug resistance and tumour microenvironment remodelling will be required to facilitate clinical application of epigenetic drugs in bladder cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249 (2021).
Magers, M. J. et al. Staging of bladder cancer. Histopathology 74, 112–134 (2019).
Burger, M. et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 63, 234–241 (2013).
Lenis, A. T., Lec, P. M., Chamie, K. & Mshs, M. D. Bladder cancer. JAMA 324, 1980–1991 https://doi.org/10.1001/jama.2020.17598 (2020).
Patel, V. G., Oh, W. K. & Galsky, M. D. Treatment of muscle‐invasive and advanced bladder cancer in 2020. CA Cancer J. Clin. 70, 404–423 (2020).
Siegel, R. L., Miller, K. D., Fuchs, H. E. & Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 72, 7–33 (2022).
Ritch, C. R. et al. Use and validation of the AUA/SUO risk grouping for nonmuscle invasive bladder cancer in a contemporary cohort. J. Urol. 203, 505–511 (2020).
van den Bosch, S. & Alfred Witjes, J. Long-term cancer-specific survival in patients with high-risk, non-muscle-invasive bladder cancer and tumour progression: a systematic review. Eur. Urol. 60, 493–500 (2011).
Dinney, C. P. et al. Focus on bladder cancer. Cancer Cell 6, 111–116 (2004).
Knowles, M. A. & Hurst, C. D. Molecular biology of bladder cancer: new insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 15, 25–41 (2015).
Stein, J. P. et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J. Clin. Oncol. 19, 666–675 (2001).
Yafi, F. A. et al. Contemporary outcomes of 2287 patients with bladder cancer who were treated with radical cystectomy: a Canadian multicentre experience. BJU Int. 108, 539–545 (2011).
Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur. Urol. 48, 202–205; discussion 205–206 (2005).
Galsky, M. D. et al. Treatment of patients with metastatic urothelial cancer “unfit” for cisplatin-based chemotherapy. J. Clin. Oncol. 29, 2432–2438 (2011).
Pietzak, E. J. et al. Next-generation sequencing of nonmuscle invasive bladder cancer reveals potential biomarkers and rational therapeutic targets. Eur. Urol. 72, 952–959 (2017).
Tran, L., Xiao, J.-F., Agarwal, N., Duex, J. E. & Theodorescu, D. Advances in bladder cancer biology and therapy. Nat. Rev. Cancer 21, 104–121 (2020).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Shen, H. & Laird, P. W. Interplay between the cancer genome and epigenome. Cell 153, 38–55 (2013).
Mills, A. A. Throwing the cancer switch: reciprocal roles of polycomb and trithorax proteins. Nat. Rev. Cancer 10, 669–682 (2010).
Allis, C. D. & Jenuwein, T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 17, 487–500 (2016).
Cheshmazar, N. et al. Current trends in development of HDAC-based chemotherapeutics. Life Sci. 308, 120946 (2022).
Pfister, S. X. & Ashworth, A. Marked for death: targeting epigenetic changes in cancer. Nat. Rev. Drug Discov. 16, 241–263 (2017).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Pinkerneil, M., Hoffmann, M. J., Schulz, W. A. & Niegisch, G. HDACs and HDAC inhibitors in urothelial carcinoma — perspectives for an antineoplastic treatment. Curr. Med. Chem. 24, 4151–4165 (2017).
Kim, K. H. & Roberts, C. W. Targeting EZH2 in cancer. Nat. Med. 22, 128–134 (2016).
Ler, L. D. et al. Loss of tumor suppressor KDM6A amplifies PRC2-regulated transcriptional repression in bladder cancer and can be targeted through inhibition of EZH2. Sci. Transl. Med. 9, eaai8312 (2017).
Andrew, R. J. Extensive heterogeneity in somatic mutation and selection in the human bladder. Science 370, 75–82 (2020).
Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Hoffmann, M. J. & Schulz, W. A. Alterations of chromatin regulators in the pathogenesis of urinary bladder urothelial carcinoma. Cancers 13, 6040 (2021).
Ozawa, A. et al. Inhibition of bladder tumour growth by histone deacetylase inhibitor. BJU Int. 105, 1181–1186 (2010).
Pinkerneil, M. et al. Inhibition of class I histone deacetylases 1 and 2 promotes urothelial carcinoma cell death by various mechanisms. Mol. Cancer Ther. 15, 299–312 (2016).
Di Cerbo, V. & Schneider, R. Cancers with wrong HATs: the impact of acetylation. Brief. Funct. Genomics 12, 231–243 (2013).
Marmorstein, R. & Zhou, M. M. Writers and readers of histone acetylation: structure, mechanism, and inhibition. Cold Spring Harb. Perspect. Biol. 6, a018762 (2014).
Koutsogiannouli, E. A. et al. Differential effects of histone acetyltransferase GCN5 or PCAF knockdown on urothelial carcinoma cells. Int. J. Mol. Sci. 18, 1449 (2017).
Jin, Q. et al. Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 (2011).
Robertson, A. G. et al. Comprehensive molecular characterization of muscle-invasive bladder cancer. Cell 171, 540–556.e525 (2017).
Kim, P. H. et al. Genomic predictors of survival in patients with high-grade urothelial carcinoma of the bladder. Eur. Urol. 67, 198–201 (2015).
Pietzak, E. J. et al. Genomic differences between “Primary” and “Secondary” muscle-invasive bladder cancer as a basis for disparate outcomes to cisplatin-based neoadjuvant chemotherapy. Eur. Urol. 75, 231–239 (2019).
Ogiwara, H. et al. Targeting p300 addiction in CBP-deficient cancers causes synthetic lethality by apoptotic cell death due to abrogation of MYC expression. Cancer Discov. 6, 430–445 (2016).
Li, J. et al. A CRISPR interference of CBP and p300 selectively induced synthetic lethality in bladder cancer cells in vitro. Int. J. Biol. Sci. 15, 1276–1286 (2019).
Ontario, D. L. et al. HATs off: selective synthetic inhibitors of the histone acetyltransferases p300 and PCAF. Mol. Cell 5, 589–595 (2000).
Rajendran, P., Williams, D. E., Ho, E. & Dashwood, R. H. Metabolism as a key to histone deacetylase inhibition. Crit. Rev. Biochem. Mol. Biol. 46, 181–199 (2011).
Alivand, M. et al. Histonedeacetylase 1 mRNA has elevated expression in clinical specimen of bladder cancer. Bratisl. Lek. Listy 119, 12–16 (2018).
Cédric, P. et al. Expression of histone deacetylases 1, 2 and 3 in urothelial bladder cancer. BMC Clin. Pathol. 13, 10 (2014).
Jurkin, J. et al. Distinct and redundant functions of histone deacetylases HDAC1 and HDAC2 in proliferation and tumorigenesis. Cell Cycle 10, 406–412 (2011).
Kelly, R. D. & Cowley, S. M. The physiological roles of histone deacetylase (HDAC) 1 and 2: complex co-stars with multiple leading parts. Biochem. Soc. Trans. 41, 741–749 (2013).
Niegisch, G. et al. Changes in histone deacetylase (HDAC) expression patterns and activity of HDAC inhibitors in urothelial cancers. Urol. Oncol. 31, 1770–1779 (2013).
Jaguva Vasudevan, A. A. et al. Proteomic and transcriptomic profiles of human urothelial cancer cells with histone deacetylase 5 overexpression. Sci. Data 9, 240 (2022).
Jaguva Vasudevan, A. A. et al. HDAC5 expression in urothelial carcinoma cell lines inhibits long-term proliferation but can promote epithelial-to-mesenchymal transition. Int. J. Mol. Sci. 20, 2135 (2019).
Butler, K. V. et al. Rational design and simple chemistry yield a superior, neuroprotective HDAC6 inhibitor, tubastatin A. J. Am. Chem. Soc. 132, 10842–10846 (2010).
Kuroki, H. et al. Histone deacetylase 6 inhibition in urothelial cancer as a potential new strategy for cancer treatment. Oncol. Lett. 21, 64 (2020).
Ota, S., Zhou, Z. Q. & Hurlin, P. J. Suppression of FGFR3- and MYC-dependent oncogenesis by tubacin: association with HDAC6-dependent and independent activities. Oncotarget 9, 3172–3187 (2018).
Rosik, L. et al. Limited efficacy of specific HDAC6 inhibition in urothelial cancer cells. Cancer Biol. Ther. 15, 742–757 (2014).
Carafa, V. et al. Sirtuin functions and modulation: from chemistry to the clinic. Clin. Epigenetics 8, 61 (2016).
Chalkiadaki, A. & Guarente, L. The multifaceted functions of sirtuins in cancer. Nat. Rev. Cancer 15, 608–624 (2015).
Monteiro-Reis, S. et al. Sirtuins’ deregulation in bladder cancer: SIRT7 is implicated in tumor progression through epithelial to mesenchymal transition promotion. Cancers 12, 1066 (2020).
Tan, P. et al. SRT1720 inhibits the growth of bladder cancer in organoids and murine models through the SIRT1-HIF axis. Oncogene 40, 6081–6092 (2021).
Chen, J., Cao, L., Li, Z. & Li, Y. SIRT1 promotes GLUT1 expression and bladder cancer progression via regulation of glucose uptake. Hum. Cell 32, 193–201 (2019).
Muller, S., Filippakopoulos, P. & Knapp, S. Bromodomains as therapeutic targets. Expert Rev. Mol. Med. 13, e29 (2011).
Filippakopoulos, P. et al. Histone recognition and large-scale structural analysis of the human bromodomain family. Cell 149, 214–231 (2012).
Zaware, N. & Zhou, M. M. Bromodomain biology and drug discovery. Nat. Struct. Mol. Biol. 26, 870–879 (2019).
Wu, X. et al. BRD4 regulates EZH2 transcription through upregulation of C-MYC and represents a novel therapeutic target in bladder cancer. Mol. Cancer Ther. 15, 1029–1042 (2016).
Liu, T., Zhang, Z., Wang, C., Huang, H. & Li, Y. BRD4 promotes the migration and invasion of bladder cancer cells through the Sonic hedgehog signaling pathway and enhances cisplatin resistance. Biochem. Cell Biol. 100, 179–187 (2022).
Huang, N., Liao, P., Zuo, Y., Zhang, L. & Jiang, R. Design, synthesis, and biological evaluation of a potent dual EZH2-BRD4 inhibitor for the treatment of some solid tumors. J. Med. Chem. 66, 2646–2662 (2023).
Hölscher, A. S., Schulz, W. A., Pinkerneil, M., Niegisch, G. & Hoffmann, M. J. Combined inhibition of BET proteins and class I HDACs synergistically induces apoptosis in urothelial carcinoma cell lines. Clin. Epigenetics 10, 1 (2018).
Martin, C. & Zhang, Y. The diverse functions of histone lysine methylation. Nat. Rev. Mol. Cell Biol. 6, 838–849 (2005).
Völkel, P. & Angrand, P. O. The control of histone lysine methylation in epigenetic regulation. Biochimie 89, 1–20 (2007).
Jenuweina, T., Laiblea, G., Dornb, R. & Reuterb, G. SET domain proteins modulate chromatin domains in eu- and heterochromatin. Cell Mol. Life Sci. 54, 80–93 (1998).
Min, J., Feng, Q., Li, Z., Zhang, Y. & Xu, R. M. Structure of the catalytic domain of human DOT1L, a non-SET domain nucleosomal histone methyltransferase. Cell 112, 711–723 (2003).
Li, Y., Ge, K., Li, T., Cai, R. & Chen, Y. The engagement of histone lysine methyltransferases with nucleosomes: structural basis, regulatory mechanisms, and therapeutic implications. Protein Cell 14, 165–179 (2023).
Wang, C. et al. Enhancer priming by H3K4 methyltransferase MLL4 controls cell fate transition. Proc. Natl Acad. Sci. USA 113, 11871–11876 (2016).
Rampias, T. et al. The lysine-specific methyltransferase KMT2C/MLL3 regulates DNA repair components in cancer. EMBO Rep. 20, e46821 (2019).
Sun, P. et al. KMT2D inhibits the growth and metastasis of bladder cancer cells by maintaining the tumor suppressor genes. Biomed. Pharmacother. 115, 108924 (2019).
Lin-Shiao, E. et al. KMT2D regulates p63 target enhancers to coordinate epithelial homeostasis. Genes Dev. 32, 181–193 (2018).
Baker, S. C., Mason, A. S. & Southgate, J. Does a novel mutagenic process target KMT2D mutation in the most common first event on the path to bladder cancer? Eur. Urol. 79, 435–436 (2021).
Li, R. et al. Macroscopic somatic clonal expansion in morphologically normal human urothelium. Science 370, 82–89 (2020).
Rozen, S. G. Mutational selection in normal urothelium. Science 370, 34–35 (2020).
Xu, J. et al. KMT2D deficiency promotes myeloid leukemias which is vulnerable to ribosome biogenesis inhibition. Adv. Sci. 10, e2206098 (2023).
Margueron, R. & Reinberg, D. The polycomb complex PRC2 and its mark in life. Nature 469, 343–349 (2011).
McCabe, M. T. et al. Mutation of A677 in histone methyltransferase EZH2 in human B-cell lymphoma promotes hypertrimethylation of histone H3 on lysine 27 (H3K27). Proc. Natl Acad. Sci. USA 109, 2989–2994 (2012).
Andrea Piunti et al. Immune activation is essential for the antitumor activity of EZH2 inhibition in urothelial carcinoma. Sci. Adv. 8, eabo8043 (2022).
Fantini, D. et al. A Carcinogen-induced mouse model recapitulates the molecular alterations of human muscle invasive bladder cancer. Oncogene 37, 1911–1925 (2018).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03854474 (2023).
Tachibana, M., Sugimoto, K., Fukushima, T. & Shinkai, Y. Set domain-containing protein, G9a, is a novel lysine-preferring mammalian histone methyltransferase with hyperactivity and specific selectivity to lysines 9 and 27 of histone H3. J. Biol. Chem. 276, 25309–25317 (2001).
Casciello, F., Windloch, K., Gannon, F. & Lee, J. S. Functional role of G9a histone methyltransferase in cancer. Front. Immunol. 6, 487 (2015).
Li, F. et al. G9a inhibition induces autophagic cell death via AMPK/mTOR pathway in bladder transitional cell carcinoma. PLoS ONE 10, e0138390 (2015).
Cao, Y. P. et al. Inhibition of G9a by a small molecule inhibitor, UNC0642, induces apoptosis of human bladder cancer cells. Acta Pharmacol. Sin. 40, 1076–1084 (2019).
San Jose-Eneriz, E. et al. Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat. Commun. 8, 15424 (2017).
Segovia, C. et al. Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat. Med. 25, 1073–1081 (2019).
Cristina, S. et al. Opposing roles of PIK3CA gene alterations to EZH2 signaling in non-muscle invasive bladder cancer. Oncotarget 8, 10531–10542 (2017).
Hu, G., Wang, X., Han, Y. & Wang, P. Protein arginine methyltransferase 5 promotes bladder cancer growth through inhibiting NF-kB dependent apoptosis. EXCLI J. 17, 1157–1166 (2018).
Liu, S. et al. Flavokawain A is a natural inhibitor of PRMT5 in bladder cancer. J. Exp. Clin. Cancer Res. 41, 293 (2022).
Shi, Y. et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 119, 941–953 (2004).
Hayami, S. et al. Overexpression of LSD1 contributes to human carcinogenesis through chromatin regulation in various cancers. Int. J. Cancer 128, 574–586 (2011).
Metzger, E. et al. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature 437, 436–439 (2005).
Kauffman, E. C. et al. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Mol. Carcinog. 50, 931–944 (2011).
Xie, Q. et al. LSD1 promotes bladder cancer progression by upregulating LEF1 and enhancing EMT. Front. Oncol. 10, 1234 (2020).
Wan, W. et al. Histone demethylase JMJD1A promotes urinary bladder cancer progression by enhancing glycolysis through coactivation of hypoxia inducible factor 1α. Oncogene 36, 3868–3877 (2017).
Cho, H. S. et al. The JmjC domain-containing histone demethylase KDM3A is a positive regulator of the G1/S transition in cancer cells via transcriptional regulation of the HOXA1 gene. Int. J. Cancer 131, E179–E189 (2012).
Pearson, J. C., Lemons, D. & McGinnis, W. Modulating Hox gene functions during animal body patterning. Nat. Rev. Genet. 6, 893–904 (2005).
Chen, S. et al. HOXA1 promotes proliferation and metastasis of bladder cancer by enhancing SMAD3 transcription. Pathol. Res. Pract. 239, 154141 (2022).
Belpaire, M., Taminiau, A., Geerts, D. & Rezsohazy, R. HOXA1, a breast cancer oncogene. Biochim. Biophys. Acta Rev. Cancer 1877, 188747 (2022).
Berry, W. L. & Janknecht, R. KDM4/JMJD2 histone demethylases: epigenetic regulators in cancer cells. Cancer Res. 73, 2936–2942 (2013).
Kogure, M. et al. Deregulation of the histone demethylase JMJD2A is involved in human carcinogenesis through regulation of the G1/S transition. Cancer Lett. 336, 76–84 (2013).
Wang, F. et al. Upregulation of JMJD2A promotes migration and invasion in bladder cancer through regulation of SLUG. Oncol. Rep. 42, 1431–1440 (2019).
Hong, S. et al. Identification of JmjC domain-containing UTX and JMJD3 as histone H3 lysine 27 demethylases. Proc. Natl Acad. Sci. USA 104, 18439–18444 (2007).
Shilatifard, A. The COMPASS family of histone H3K4 methylases: mechanisms of regulation in development and disease pathogenesis. Annu. Rev. Biochem. 81, 65–95 (2012).
Nickerson, M. L. et al. Concurrent alterations in TERT, KDM6A, and the BRCA pathway in bladder cancer. Clin. Cancer Res. 20, 4935–4948 (2014).
Gui, Y. et al. Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat. Genet. 43, 875–878 (2011).
Nassar, A. H. et al. Mutational analysis of 472 urothelial carcinoma across grades and anatomic sites. Clin. Cancer Res. 25, 2458–2470 (2019).
Kaneko, S., Li, X. X chromosome protects against bladder cancer in females via a KDM6A-dependent epigenetic mechanism. Sci. Adv. 4, eaar5598 (2018).
van Haaften, G. et al. Somatic mutations of the histone H3K27 demethylase gene UTX in human cancer. Nat. Genet. 41, 521–523 (2009).
Liu, L. et al. KDM6A-ARHGDIB axis blocks metastasis of bladder cancer by inhibiting Rac1. Mol. Cancer 20, 77 (2021).
Yimin, W. et al. Src phosphorylation of RhoGDI2 regulates its metastasis suppressor function. Proc. Natl Acad. Sci. USA 106, 5807–5812 (2009).
Kong, N. et al. Intravesical delivery of KDM6A-mRNA via mucoadhesive nanoparticles inhibits the metastasis of bladder cancer. Proc. Natl Acad. Sci. USA 119, e2112696119 (2022).
Agger, K. et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 449, 731–734 (2007).
Ramakrishnan, S. et al. Inhibition of EZH2 induces NK cell-mediated differentiation and death in muscle-invasive bladder cancer. Cell Death Differ. 26, 2100–2114 (2019).
Kobatake, K. et al. Kdm6a deficiency activates inflammatory pathways, promotes M2 macrophage polarization, and causes bladder cancer in cooperation with p53 dysfunction. Clin. Cancer Res. 26, 2065–2079 (2020).
Goswami, K. K. et al. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 316, 1–10 (2017).
Mantovani, A., Allavena, P., Marchesi, F. & Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 21, 799–820 (2022).
Chaturvedi, S. S., Ramanan, R., Waheed, S. O., Karabencheva-Christova, T. G. & Christov, C. Z. Structure-function relationships in KDM7 histone demethylases. Adv. Protein Chem. Struct. Biol. 117, 113–125 (2019).
Lee, K. H. et al. Histone demethylase KDM7A regulates androgen receptor activity, and its chemical inhibitor TC-E 5002 overcomes cisplatin-resistance in bladder cancer cells. Int. J. Mol. Sci. 21, 5658 (2020).
Lee, K. H. et al. Histone demethylase KDM7A controls androgen receptor activity and tumor growth in prostate cancer. Int. J. Cancer 143, 2849–2861 (2018).
Chen, J. et al. The androgen receptor in bladder cancer. Nat. Rev. Urol. 20, 560–574 (2023).
Lee, J. H., Tate, C. M., You, J. S. & Skalnik, D. G. Identification and characterization of the human Set1B histone H3-Lys4 methyltransferase complex. J. Biol. Chem. 282, 13419–13428 (2007).
Lu, K., Tao, H., Si, X. & Chen, Q. The histone H3 lysine 4 presenter WDR5 as an oncogenic protein and novel epigenetic target in cancer. Front. Oncol. 8, 502 (2018).
Chen, X. et al. Upregulated WDR5 promotes proliferation, self-renewal and chemoresistance in bladder cancer via mediating H3K4 trimethylation. Sci. Rep. 5, 8293 (2015).
Grebien, F. et al. Pharmacological targeting of the Wdr5-MLL interaction in C/EBPα N-terminal leukemia. Nat. Chem. Biol. 11, 571–578 (2015).
Zhang, J. et al. Targeting WD repeat domain 5 enhances chemosensitivity and inhibits proliferation and programmed death-ligand 1 expression in bladder cancer. J. Exp. Clin. Cancer Res. 40, 203 (2021).
Huang, X. et al. Targeting epigenetic crosstalk as a therapeutic strategy for EZH2-aberrant solid tumors. Cell 175, 186–199 e119 (2018).
Mohammad, H. P., Barbash, O. & Creasy, C. L. Targeting epigenetic modifications in cancer therapy: erasing the roadmap to cancer. Nat. Med. 25, 403–418 (2019).
Pan, D. et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 359, 770–775 (2018).
Ghorani, E. & Quezada, S. A. Chromatin regulation and immune escape. Science 359, 745–746 (2018).
Juergens, R. A. et al. Combination epigenetic therapy has efficacy in patients with refractory advanced non-small cell lung cancer. Cancer Discov. 1, 598–607 (2011).
Chou, T. C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res 70, 440–446 (2010).
Zheng, H. et al. HDAC inhibitors enhance T-cell chemokine expression and augment response to PD-1 immunotherapy in lung adenocarcinoma. Clin. Cancer Res. 22, 4119–4132 (2016).
Kim, K. et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc. Natl Acad. Sci. USA 111, 11774–11779 (2014).
Orillion, A. et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin. Cancer Res. 23, 5187–5201 (2017).
Woods, D. M. et al. HDAC inhibition upregulates PD-1 ligands in melanoma and augments immunotherapy with PD-1 blockade. Cancer Immunol. Res. 3, 1375–1385 (2015).
Stone, M. L. et al. Epigenetic therapy activates type I interferon signaling in murine ovarian cancer to reduce immunosuppression and tumor burden. Proc. Natl Acad. Sci. USA 114, E10981–e10990 (2017).
Llopiz, D. et al. Enhanced anti-tumor efficacy of checkpoint inhibitors in combination with the histone deacetylase inhibitor Belinostat in a murine hepatocellular carcinoma model. Cancer Immunol. Immunother. 68, 379–393 (2019).
Knox, T. et al. Selective HDAC6 inhibitors improve anti-PD-1 immune checkpoint blockade therapy by decreasing the anti-inflammatory phenotype of macrophages and down-regulation of immunosuppressive proteins in tumor cells. Sci. Rep. 9, 6136 (2019).
Briere, D. et al. The class I/IV HDAC inhibitor mocetinostat increases tumor antigen presentation, decreases immune suppressive cell types and augments checkpoint inhibitor therapy. Cancer Immunol. Immunother. 67, 381–392 (2018).
Cycon, K. A., Mulvaney, K., Rimsza, L. M., Persky, D. & Murphy, S. P. Histone deacetylase inhibitors activate CIITA and MHC class II antigen expression in diffuse large B-cell lymphoma. Immunology 140, 259–272 (2013).
Jensen, H., Andresen, L., Hansen, K. A. & Skov, S. Cell-surface expression of Hsp70 on hematopoietic cancer cells after inhibition of HDAC activity. J. Leukoc. Biol. 86, 923–932 (2009).
Burke, B. et al. Inhibition of histone deacetylase (HDAC) enhances checkpoint blockade efficacy by rendering bladder cancer cells visible for T cell-mediated destruction. Front. Oncol. 10, 699 (2020).
Saito, R. et al. Molecular subtype-specific immunocompetent models of high-grade urothelial carcinoma reveal differential neoantigen expression and response to immunotherapy. Cancer Res. 78, 3954–3968 (2018).
Truong, A. S. et al. Entinostat induces antitumor immune responses through immune editing of tumor neoantigens. J. Clin. Invest. 131, e138560 (2021).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00045006 (2013).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00413075 (2015).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00363883 (2015).
Grivas, P. et al. Mocetinostat for patients with previously treated, locally advanced/metastatic urothelial carcinoma and inactivating alterations of acetyltransferase genes. Cancer 125, 533–540 (2019).
US ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT02619253 (2023).
Roberto, P. et al. Immunomodulation by HDAC inhibition: results from a phase Ib study with vorinostat and pembrolizumab in metastatic urothelial, renal, and prostate carcinoma patients. J. Clin. Oncol. 37, 2572–2572 (2019).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00565227 (2016).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT03978624 (2023).
ClinicalTrials.gov. US National Library of Medicine. https://clinicaltrials.gov/ct2/show/NCT00421889 (2015).
Leslie, M. First EZH2 inhibitor approved — for rare sarcoma. Cancer Discov. 10, 333–334 (2020).
Joshua, J. et al. A pilot study of tazemetostat and MK-3475 (pembrolizumab) in advanced urothelial carcinoma (ETCTN 10183). J. Clin. Oncol. 38, TPS607 (2020).
Audia, J. E. & Campbell, R. M. Histone modifications and cancer. Cold Spring Harb. Perspect. Biol. 8, a019521 (2016).
Chuikov, S. et al. Regulation of p53 activity through lysine methylation. Nature 432, 353–360 (2004).
Kim, E. et al. Phosphorylation of EZH2 activates STAT3 signaling via STAT3 methylation and promotes tumorigenicity of glioblastoma stem-like cells. Cancer Cell 23, 839–852 (2013).
Kexin, X. et al. Oncogenic activity in castration-resistant prostate cancer cells is polycomb-independent. Science 338, 1465–1469 (2012).
Jung, H. Y. et al. PAF and EZH2 induce Wnt/β-catenin signaling hyperactivation. Mol. Cell 52, 193–205 (2013).
Gonzalez, M. E. et al. EZH2 expands breast stem cells through activation of NOTCH1 signaling. Proc. Natl Acad. Sci. USA 111, 3098–3103 (2014).
Kim, K. H. et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat. Med. 21, 1491–1496 (2015).
Flavahan, W. A., Gaskell, E. & Bernstein, B. E. Epigenetic plasticity and the hallmarks of cancer. Science 357, eaal2380 (2017).
Acknowledgements
This work was supported by the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University ZYGD23001 and Sichuan Science and Technology Program (2023NSFSC1905).
Author information
Authors and Affiliations
Contributions
S.Z. and X.X. researched data for the article. S.Z., T.L. and P.T. contributed substantially to discussion of the content. S.Z. wrote the article. Q.W., S.Z., C.C. and P.T. reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Urology thanks A. Sato, W. Schulz and F. Khanim for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, S., Lin, T., Xiong, X. et al. Targeting histone modifiers in bladder cancer therapy — preclinical and clinical evidence. Nat Rev Urol (2024). https://doi.org/10.1038/s41585-024-00857-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41585-024-00857-z