Abstract
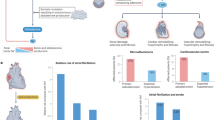
Early diagnosis and appropriate treatment of primary aldosteronism, the most frequent cause of secondary hypertension, are crucial to prevent deleterious cardiovascular outcomes. In the past decade, the discovery of genetic abnormalities responsible for sporadic and familial forms of primary aldosteronism has improved the knowledge of the pathogenesis of this disorder. Mutations in genes encoding ion channels and pumps lead to increased cytosolic concentrations of calcium in zona glomerulosa cells, which triggers CYP11B2 expression and autonomous aldosterone production. Improved understanding of the mechanisms underlying the disease is key to improving diagnostics and to developing and implementing targeted treatments. This Review provides an update on the genetic abnormalities associated with sporadic and familial forms of primary aldosteronism, their frequency among different populations and the mechanisms explaining excessive aldosterone production and adrenal nodule development. The possible effects and uses of these findings for improving the diagnostics for primary aldosteronism are discussed. Furthermore, current treatment options of primary aldosteronism are reviewed, with particular attention to the latest studies on blood pressure and cardiovascular outcomes following medical or surgical treatment. The new perspectives regarding the use of targeted drug therapy for aldosterone-producing adenomas with specific somatic mutations are also addressed.
Key points
-
Primary aldosteronism is the most frequent form of secondary hypertension and is curable.
-
The condition is largely underdiagnosed, preventing patients from receiving targeted treatment and preventing cardiovascular complications.
-
Different genetic abnormalities have been identified in aldosterone-producing adenoma and familial forms of the disease.
-
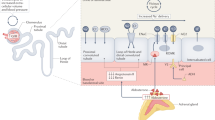
Most genetic abnormalities increase intracellular calcium signalling in the adrenal zona glomerulosa, increasing aldosterone production.
-
New approaches are currently being developed to achieve more rapid and precise diagnosis of the condition and for more efficient targeted treatment.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Monticone, S. et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J. Am. Coll. Cardiol. 69, 1811–1820 (2017).
Hannemann, A. & Wallaschofski, H. Prevalence of primary aldosteronism in patient’s cohorts and in population-based studies — a review of the current literature. Horm. Metab. Res. 44, 157–162 (2012).
Brown, J. M. et al. The unrecognized prevalence of primary aldosteronism: a cross-sectional study. Ann. Intern. Med. 173, 10–20 (2020).
Funder, J. W. et al. The management of primary aldosteronism: case detection, diagnosis, and treatment: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 101, 1889–1916 (2016).
Mulatero, P. et al. Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms). Hypertension 58, 797–803 (2011).
Lifton, R. P. et al. A chimaeric 11beta-hydroxylase aldosterone synthase gene causes glucocorticoid-remediable aldosteronism and human hypertension. Nature 355, 262–265 (1992).
Choi, M. et al. K+ channel mutations in adrenal aldosterone-producing adenomas and hereditary hypertension. Science 331, 768–772 (2011).
Scholl, U. I. et al. Recurrent gain of function mutation in calcium channel CACNA1H causes early-onset hypertension with primary aldosteronism. eLife 4, e06315 (2015).
Scholl, U. I. et al. CLCN2 chloride channel mutations in familial hyperaldosteronism type II. Nat. Genet. 50, 349–354 (2018).
Fernandes-Rosa, F. L. et al. A gain-of-function mutation in the CLCN2 chloride channel gene causes primary aldosteronism. Nat. Genet. 50, 355–361 (2018).
Rossier, B. C., Baker, M. E. & Studer, R. A. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol. Rev. 95, 297–340 (2015).
Savard, S., Amar, L., Plouin, P. F. & Steichen, O. Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study. Hypertension 62, 331–336 (2013).
Monticone, S. et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 6, 41–50 (2018).
Milliez, P. et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 45, 1243–1248 (2005).
Rossi, G. P. et al. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol. Metab. 19, 88–90 (2008).
Rossi, G. P. et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J. Am. Coll. Cardiol. 48, 2293–2300 (2006).
Born-Frontsberg, E. et al. Cardiovascular and cerebrovascular comorbidities of hypokalemic and normokalemic primary aldosteronism: results of the German Conn’s Registry. J. Clin. Endocrinol. Metab. 94, 1125–1130 (2009).
Rossi, G. P. et al. Remodeling of the left ventricle in primary aldosteronism due to Conn’s adenoma. Circulation 95, 1471–1478 (1997).
Freel, E. M. et al. Demonstration of blood pressure-independent noninfarct myocardial fibrosis in primary aldosteronism: a cardiac magnetic resonance imaging study. Circ. Cardiovasc. Imaging 5, 740–747 (2012).
Leopold, J. A. et al. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat. Med. 13, 189–197 (2007).
Brown, N. J. Aldosterone and end-organ damage. Curr. Opin. Nephrol. Hypertens. 14, 235–241 (2005).
Calhoun, D. A. Hyperaldosteronism as a common cause of resistant hypertension. Annu. Rev. Med. 64, 233–247 (2013).
Young, W. F. Jr. Diagnosis and treatment of primary aldosteronism: practical clinical perspectives. J. Intern. Med. 285, 126–148 (2019).
Nishikawa, T. et al. Guidelines for the diagnosis and treatment of primary aldosteronism — the Japan Endocrine Society 2009. Endocr. J. 58, 711–721 (2011).
Eisenhofer, G. et al. Mass spectrometry-based adrenal and peripheral venous steroid profiling for subtyping primary aldosteronism. Clin. Chem. 62, 514–524 (2016).
Turcu, A. F. et al. Comprehensive analysis of steroid biomarkers for guiding primary aldosteronism subtyping. Hypertension 75, 183–192 (2020).
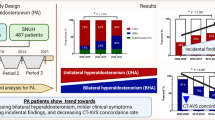
Dekkers, T. et al. Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial. Lancet Diabetes Endocrinol. 4, 739–746 (2016).
Rossi, G. P. & Funder, J. W. Adrenal venous sampling versus computed tomographic scan to determine treatment in primary aldosteronism (the SPARTACUS trial): a critique. Hypertension 69, 396–397 (2017).
Williams, T. A. et al. Computed tomography and adrenal venous sampling in the diagnosis of unilateral primary aldosteronism. Hypertension 72, 641–649 (2018).
Rossi, G. P. et al. Clinical outcomes of 1625 patients with primary aldosteronism subtyped with adrenal vein sampling. Hypertension 74, 800–808 (2019).
Stowasser, M. et al. Familial hyperaldosteronism type II: five families with a new variety of primary aldosteronism. Clin. Exp. Pharmacol. Physiol. 19, 319–322 (1992).
Geller, D. S. et al. A novel form of human Mendelian hypertension featuring nonglucocorticoid-remediable aldosteronism. J. Clin. Endocrinol. Metab. 93, 3117–3123 (2008).
Sutherland, D. J., Ruse, J. L. & Laidlaw, J. C. Hypertension, increased aldosterone secretion and low plasma renin activity relieved by dexamethasone. Can. Med. Assoc. J. 95, 1109–1119 (1966).
Halperin, F. & Dluhy, R. G. Glucocorticoid-remediable aldosteronism. Endocrinol. Metab. Clin. North Am. 40, 333–341 (2011).
Pascoe, L. et al. Glucocorticoid-suppressible hyperaldosteronism results from hybrid genes created by unequal crossovers between CYP11B1 and CYP11B2. Proc. Natl Acad. Sci. USA 89, 8327–8331 (1992).
Pascoe, L. et al. Glucocorticoid-suppressible hyperaldosteronism and adrenal tumors occurring in a single French pedigree. J. Clin. Invest. 96, 2236–2246 (1995).
Stowasser, M. & Gordon, R. D. Primary aldosteronism: learning from the study of familial varieties. J. Hypertens. 18, 1165–1176 (2000).
Mulatero, P. et al. Recombinant CYP11B genes encode enzymes that can catalyze conversion of 11-deoxycortisol to cortisol, 18-hydroxycortisol, and 18-oxocortisol. J. Clin. Endocrinol. Metab. 83, 3996–4001 (1998).
Mulatero, P. et al. Diagnosis of glucocorticoid-remediable aldosteronism in primary aldosteronism: aldosterone response to dexamethasone and long polymerase chain reaction for chimeric gene. J. Clin. Endocrinol. Metab. 83, 2573–2575 (1998).
Stowasser, M. & Gordon, R. D. Familial hyperaldosteronism. J. Steroid Biochem. Mol. Biol. 78, 215–229 (2001).
Medeau, V. et al. Familial aspect of primary hyperaldosteronism: analysis of families compatible with primary hyperaldosteronism type 2. Ann. Endocrinol. 66, 240–246 (2005).
Pallauf, A. et al. The prevalence of familial hyperaldosteronism in apparently sporadic primary aldosteronism in Germany: a single center experience. Horm. Metab. Res. 44, 215–220 (2012).
Mulatero, P. et al. KCNJ5 mutations in European families with nonglucocorticoid remediable familial hyperaldosteronism. Hypertension 59, 235–240 (2012).
Daniil, G. et al. CACNA1H mutations are associated with different forms of primary aldosteronism. EBioMedicine 13, 225–236 (2016).
Perez-Rivas, L. G., Williams, T. A. & Reincke, M. Inherited forms of primary hyperaldosteronism: new genes, new phenotypes and proposition of a new classification. Exp. Clin. Endocrinol. Diabetes 127, 93–99 (2019).
Mulatero, P., Monticone, S., Rainey, W. E., Veglio, F. & Williams, T. A. Role of KCNJ5 in familial and sporadic primary aldosteronism. Nat. Rev. Endocrinol. 9, 104–112 (2013).
Scholl, U. I. et al. Hypertension with or without adrenal hyperplasia due to different inherited mutations in the potassium channel KCNJ5. Proc. Natl Acad. Sci. USA 109, 2533–2538 (2012).
Monticone, S. et al. A novel Y152C KCNJ5 mutation responsible for familial hyperaldosteronism type III. J. Clin. Endocrinol. Metab. 98, E1861–E1865 (2013).
Adachi, M. et al. Discordant genotype–phenotype correlation in familial hyperaldosteronism type III with KCNJ5 gene mutation: a patient report and review of the literature. Horm. Res. Paediatr. 82, 138–142 (2014).
Gomez-Sanchez, C. E. et al. Disordered zonal and cellular CYP11B2 enzyme expression in familial hyperaldosteronism type 3. Mol. Cell Endocrinol. 439, 74–80 (2017).
Tong, A. et al. A novel phenotype of familial hyperaldosteronism type III: concurrence of aldosteronism and Cushing’s syndrome. J. Clin. Endocrinol. Metab. 101, 4290–4297 (2016).
Scholl, U. I. et al. Somatic and germline CACNA1D calcium channel mutations in aldosterone-producing adenomas and primary aldosteronism. Nat. Genet. 45, 1050–1054 (2013).
Semenova, N. A., Ryzhkova, O. R., Strokova, T. V. & Taran, N. N. The third case report a patient with primary aldosteronism, seizures, and neurologic abnormalities (PASNA) syndrome de novo variant mutations in the CACNA1D gene [Russian]. Zh Nevrol. Psikhiatr Im. S S Korsakova 118, 49–52 (2018).
De Mingo Alemany, M. C., Mifsud Grau, L., Moreno Macian, F., Ferrer Lorente, B. & Leon Carinena, S. A de novo CACNA1D missense mutation in a patient with congenital hyperinsulinism, primary hyperaldosteronism and hypotonia. Channels 14, 175–180 (2020).
Flanagan, S. E. et al. A CACNA1D mutation in a patient with persistent hyperinsulinaemic hypoglycaemia, heart defects, and severe hypotonia. Pediatr. Diabetes 18, 320–323 (2017).
Dekkers, T. et al. Adrenal nodularity and somatic mutations in primary aldosteronism: one node is the culprit? J. Clin. Endocrinol. Metab. 99, E1341–E1351 (2014).
Omura, M., Sasano, H., Fujiwara, T., Yamaguchi, K. & Nishikawa, T. Unique cases of unilateral hyperaldosteronemia due to multiple adrenocortical micronodules, which can only be detected by selective adrenal venous sampling. Metabolism 51, 350–355 (2002).
Monticone, S. et al. Immunohistochemical, genetic and clinical characterization of sporadic aldosterone-producing adenomas. Mol. Cell Endocrinol. 411, 146–154 (2015).
Fernandes-Rosa, F. L. et al. Different somatic mutations in multinodular adrenals with aldosterone-producing adenoma. Hypertension 66, 1014–1022 (2015).
Yamazaki, Y. et al. Histopathological classification of cross-sectional image-negative hyperaldosteronism. J. Clin. Endocrinol. Metab. 102, 1182–1192 (2017).
Azizan, E. A. et al. Somatic mutations in ATP1A1 and CACNA1D underlie a common subtype of adrenal hypertension. Nat. Genet. 45, 1055–1060 (2013).
Beuschlein, F. et al. Somatic mutations in ATP1A1 and ATP2B3 lead to aldosterone-producing adenomas and secondary hypertension. Nat. Genet. 45, 440–444 (2013).
Fernandes-Rosa, F. L. et al. Genetic spectrum and clinical correlates of somatic mutations in aldosterone-producing adenoma. Hypertension 64, 354–361 (2014).
Lenzini, L. et al. A meta-analysis of somatic KCNJ5 K+ channel mutations in 1636 patients with an aldosterone-producing adenoma. J. Clin. Endocrinol. Metab. 100, E1089–E1095 (2015).
Zennaro, M. C., Boulkroun, S. & Fernandes-Rosa, F. Genetic causes of functional adrenocortical adenomas. Endocr. Rev. 38, 516–537 (2017).
Scholl, U. I. et al. Novel somatic mutations in primary hyperaldosteronism are related to the clinical, radiological and pathological phenotype. Clin. Endocrinol. 83, 779–789 (2015).
Akerstrom, T. et al. Activating mutations in CTNNB1 in aldosterone producing adenomas. Sci. Rep. 6, 19546 (2016).
Teo, A. E. et al. Pregnancy, primary aldosteronism, and adrenal CTNNB1 mutations. N. Engl. J. Med. 373, 1429–1436 (2015).
Rhayem, Y. et al. PRKACA somatic mutations are rare findings in aldosterone-producing adenomas. J. Clin. Endocrinol. Metab. 101, 3010–3017 (2016).
Tadjine, M., Lampron, A., Ouadi, L. & Bourdeau, I. Frequent mutations of beta-catenin gene in sporadic secreting adrenocortical adenomas. Clin. Endocrinol. 68, 264–270 (2008).
Tissier, F. et al. Mutations of beta-catenin in adrenocortical tumors: activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 65, 7622–7627 (2005).
Beuschlein, F. et al. Constitutive activation of PKA catalytic subunit in adrenal Cushing’s syndrome. N. Engl. J. Med. 370, 1019–1028 (2014).
Cao, Y. et al. Activating hotspot L205R mutation in PRKACA and adrenal Cushing’s syndrome. Science 344, 913–917 (2014).
Sato, Y. et al. Recurrent somatic mutations underlie corticotropin-independent Cushing’s syndrome. Science 344, 917–920 (2014).
Goh, G. et al. Recurrent activating mutation in PRKACA in cortisol-producing adrenal tumors. Nat. Genet. 46, 613–617 (2014).
Nanba, K. et al. Targeted molecular characterization of aldosterone-producing adenomas in white Americans. J. Clin. Endocrinol. Metab. 103, 3869–3876 (2018).
Nanba, K. et al. Genetic characteristics of aldosterone-producing adenomas in blacks. Hypertension 73, 885–892 (2019).
De Sousa, K. et al. Genetic, cellular, and molecular heterogeneity in adrenals with aldosterone-producing adenoma. Hypertension 75, 1034–1044 (2020).
Nishimoto, K. et al. Aldosterone-stimulating somatic gene mutations are common in normal adrenal glands. Proc. Natl Acad. Sci. USA 112, E4591–E4599 (2015).
Omata, K. et al. Cellular and genetic causes of idiopathic hyperaldosteronism. Hypertension 72, 874–880 (2018).
Spat, A. & Hunyady, L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol. Rev. 84, 489–539 (2004).
Oki, K., Plonczynski, M. W., Lam, M. L., Gomez-Sanchez, E. P. & Gomez-Sanchez, C. E. The potassium channel, Kir3.4, participates in angiotensin II-stimulated aldosterone production by a human adrenocortical cell line. Endocrinology 153, 4328–4335 (2012).
Murthy, M., Azizan, E. A., Brown, M. J. & O’Shaughnessy, K. M. Characterization of a novel somatic KCNJ5 mutation delI157 in an aldosterone-producing adenoma. J. Hypertens. 30, 1827–1833 (2012).
Cheng, C. J. et al. Novel KCNJ5 mutations in sporadic aldosterone-producing adenoma reduce Kir3.4 membrane abundance. J. Clin. Endocrinol. Metab. 100, E155–E163 (2015).
Yang, Y. et al. Primary aldosteronism: KCNJ5 mutations and adrenocortical cell growth. Hypertension 74, 809–816 (2019).
Jentsch, T. J. & Pusch, M. CLC chloride channels and transporters: structure, function, physiology, and disease. Physiol. Rev. 98, 1493–1590 (2018).
Grunder, S., Thiemann, A., Pusch, M. & Jentsch, T. J. Regions involved in the opening of CIC-2 chloride channel by voltage and cell volume. Nature 360, 759–762 (1992).
Dutta, R. K. et al. A somatic mutation in CLCN2 identified in a sporadic aldosterone-producing adenoma. Eur. J. Endocrinol. 181, K37–K41 (2019).
Goppner, C. et al. Pathogenesis of hypertension in a mouse model for human CLCN2 related hyperaldosteronism. Nat. Commun. 10, 4678 (2019).
Schewe, J. et al. Elevated aldosterone and blood pressure in a mouse model of familial hyperaldosteronism with ClC-2 mutation. Nat. Commun. 10, 5155 (2019).
Stindl, J. et al. Pathogenesis of adrenal aldosterone-producing adenomas carrying mutations of the Na+/K+-ATPase. Endocrinology 156, 4582–4591 (2015).
Kaplan, J. H. Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 (2002).
Williams, T. A. et al. Somatic ATP1A1, ATP2B3, and KCNJ5 mutations in aldosterone-producing adenomas. Hypertension 63, 188–195 (2014).
Zheng, F. F. et al. Clinical characteristics of somatic mutations in Chinese patients with aldosterone-producing adenoma. Hypertension 65, 622–628 (2015).
Akerstrom, T. et al. Novel somatic mutations and distinct molecular signature in aldosterone-producing adenomas. Endocr. Relat. Cancer 22, 735–744 (2015).
Einholm, A. P., Andersen, J. P. & Vilsen, B. Importance of Leu99 in transmembrane segment M1 of the Na+, K+-ATPase in the binding and occlusion of K+. J. Biol. Chem. 282, 23854–23866 (2007).
Dutta, R. K. et al. Complementary somatic mutations of KCNJ5, ATP1A1, and ATP2B3 in sporadic aldosterone producing adrenal adenomas. Endocr. Relat. Cancer 21, L1–L4 (2014).
Tauber, P. et al. Cellular pathophysiology of an adrenal adenoma-associated mutant of the plasma membrane Ca2+-ATPase ATP2B3. Endocrinology 157, 2489–2499 (2016).
Nishimoto, K. et al. Case report: nodule development from subcapsular aldosterone-producing cell clusters causes hyperaldosteronism. J. Clin. Endocrinol. Metab. 101, 6–9 (2016).
Nishimoto, K. et al. Adrenocortical zonation in humans under normal and pathological conditions. J. Clin. Endocrinol. Metab. 95, 2296–2305 (2010).
Boulkroun, S. et al. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension 56, 885–892 (2010).
Nishimoto, K. et al. Immunohistochemistry of aldosterone synthase leads the way to the pathogenesis of primary aldosteronism. Mol. Cell Endocrinol. 441, 124–133 (2017).
Sun, N. et al. Mass spectrometry imaging establishes 2 distinct metabolic phenotypes of aldosterone-producing cell clusters in primary aldosteronism. Hypertension 75, 634–644 (2020).
Boulkroun, S. et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology 152, 4753–4763 (2011).
Berthon, A. et al. WNT/beta-catenin signalling is activated in aldosterone-producing adenomas and controls aldosterone production. Hum. Mol. Genet. 23, 889–905 (2014).
Enberg, U. et al. Postoperative differentiation between unilateral adrenal adenoma and bilateral adrenal hyperplasia in primary aldosteronism by mRNA expression of the gene CYP11B2. Eur. J. Endocrinol. 151, 73–85 (2004).
Vouillarmet, J. et al. Aldosterone-producing adenoma with a somatic KCNJ5 mutation revealing APC-dependent familial adenomatous polyposis. J. Clin. Endocrinol. Metab. 101, 3874–3878 (2016).
Stowasser, M. et al. Evidence for abnormal left ventricular structure and function in normotensive individuals with familial hyperaldosteronism type I. J. Clin. Endocrinol. Metab. 90, 5070–5076 (2005).
Tauber, P. et al. Pharmacology and pathophysiology of mutated KCNJ5 found in adrenal aldosterone-producing adenomas. Endocrinology 155, 1353–1362 (2014).
Scholl, U. I. et al. Macrolides selectively inhibit mutant KCNJ5 potassium channels that cause aldosterone-producing adenoma. J. Clin. Invest. 127, 2739–2750 (2017).
Williams, T. A. et al. Genotype-specific steroid profiles associated with aldosterone-producing adenomas. Hypertension 67, 139–145 (2016).
Osswald, A. et al. Lack of influence of somatic mutations on steroid gradients during adrenal vein sampling in aldosterone-producing adenoma patients. Eur. J. Endocrinol. 169, 657–663 (2013).
Kitamoto, T. et al. Clinical and steroidogenic characteristics of aldosterone-producing adenomas with ATPase or CACNA1D gene mutations. J. Clin. Endocrinol. Metab. 101, 494–503 (2016).
Hattangady, N. G. et al. Mutated KCNJ5 activates the acute and chronic regulatory steps in aldosterone production. J. Mol. Endocrinol. 57, 1–11 (2016).
Tezuka, Y. et al. 18-Oxocortisol synthesis in aldosterone-producing adrenocortical adenoma and significance of KCNJ5 mutation status. Hypertension 73, 1283–1290 (2019).
Murakami, M. et al. In situ metabolomics of aldosterone-producing adenomas. JCI Insight 4, e130356 (2019).
Maiolino, G. et al. Macrolides for KCNJ5-mutated aldosterone-producing adenoma (MAPA): design of a study for personalized diagnosis of primary aldosteronism. Blood Press. 27, 200–205 (2018).
Catena, C. et al. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch. Intern. Med. 168, 80–85 (2008).
Reincke, M. et al. Observational study mortality in treated primary aldosteronism: the German Conn’s registry. Hypertension 60, 618–624 (2012).
Wu, V. C. et al. Long term outcome of aldosteronism after target treatments. Sci. Rep. 6, 32103 (2016).
Sukor, N., Kogovsek, C., Gordon, R. D., Robson, D. & Stowasser, M. Improved quality of life, blood pressure, and biochemical status following laparoscopic adrenalectomy for unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 95, 1360–1364 (2010).
Steichen, O., Zinzindohoue, F., Plouin, P. F. & Amar, L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review. Horm. Metab. Res. 44, 221–227 (2012).
Rossi, G. P. et al. Long-term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension 62, 62–69 (2013).
Williams, T. A. et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 5, 689–699 (2017).
Chang, Y. H. et al. Surgery decreases the long-term incident stroke risk in patients with primary aldosteronism. Surgery 167, 367–377 (2020).
Rossi, G. P. et al. Adrenalectomy lowers incident atrial fibrillation in primary aldosteronism patients at long term. Hypertension 71, 585–591 (2018).
Lin, Y. H. et al. Adrenalectomy improves increased carotid intima-media thickness and arterial stiffness in patients with aldosterone producing adenoma. Atherosclerosis 221, 154–159 (2012).
Lin, Y. H. et al. Adrenalectomy reverses myocardial fibrosis in patients with primary aldosteronism. J. Hypertens. 30, 1606–1613 (2012).
Komada, H. et al. Insulin secretion and sensitivity before and after surgical treatment for aldosterone-producing adenoma. Diabetes Metab. 46, 236–242 (2020).
Sonino, N. et al. Psychological assessment of primary aldosteronism: a controlled study. J. Clin. Endocrinol. Metab. 96, E878–E883 (2011).
Apostolopoulou, K. et al. Gender differences in anxiety and depressive symptoms in patients with primary hyperaldosteronism: a cross-sectional study. World J. Biol. Psychiatry 15, 26–35 (2014).
Velema, M. et al. Quality of life in primary aldosteronism: a comparative effectiveness study of adrenalectomy and medical treatment. J. Clin. Endocrinol. Metab. 103, 16–24 (2018).
Citton, M., Viel, G., Torresan, F., Rossi, G. P. & Iacobone, M. Effect of unilateral adrenalectomy on the quality of life of patients with lateralized primary aldosteronism. BMC Surg. 18, 105 (2019).
Ishidoya, S. et al. Changes in quality of life after laparoscopic adrenalectomy for patients with primary aldosteronism: prospective 2-year longitudinal cohort study in a Japanese tertiary center. Int. J. Urol. 26, 752–753 (2019).
Velema, M. S. et al. A disease-specific quality of life questionnaire for primary aldosteronism. Endocr. Connect. 8, 389–397 (2019).
Morisaki, M. et al. Predictors of clinical success after surgery for primary aldosteronism in the Japanese nationwide cohort. J. Endocr. Soc. 3, 2012–2022 (2019).
Vilela, L. A. P. et al. KCNJ5 somatic mutation is a predictor of hypertension remission after adrenalectomy for unilateral primary aldosteronism. J. Clin. Endocrinol. Metab. 104, 4695–4702 (2019).
Parthasarathy, H. K. et al. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J. Hypertens. 29, 980–990 (2011).
Karagiannis, A. et al. Spironolactone versus eplerenone for the treatment of idiopathic hyperaldosteronism. Expert. Opin. Pharmacother. 9, 509–515 (2008).
Rossi, G. P. Primary aldosteronism: JACC state-of-the-art review. J. Am. Coll. Cardiol. 74, 2799–2811 (2019).
Jeunemaitre, X. et al. Efficacy and tolerance of spironolactone in essential hypertension. Am. J. Cardiol. 60, 820–825 (1987).
de Gasparo, M. et al. Three new epoxy-spirolactone derivatives: characterization in vivo and in vitro. J. Pharmacol. Exp. Ther. 240, 650–656 (1987).
Burgess, E. D. et al. Long-term safety and efficacy of the selective aldosterone blocker eplerenone in patients with essential hypertension. Clin. Ther. 25, 2388–2404 (2003).
Capelli, I. et al. New mineralocorticoid receptor antagonists: update on their use in chronic kidney disease and heart failure. J. Nephrol. 33, 37–48 (2020).
Mulatero, P. et al. Long-term cardio- and cerebrovascular events in patients with primary aldosteronism. J. Clin. Endocrinol. Metab. 98, 4826–4833 (2013).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol. 6, 51–59 (2018).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Incidence of atrial fibrillation and mineralocorticoid receptor activity in patients with medically and surgically treated primary aldosteronism. JAMA Cardiol. 3, 768–774 (2018).
Hundemer, G. L., Curhan, G. C., Yozamp, N., Wang, M. & Vaidya, A. Renal outcomes in medically and surgically treated primary aldosteronism. Hypertension 72, 658–666 (2018).
Vaidya, A., Mulatero, P., Baudrand, R. & Adler, G. K. The expanding spectrum of primary aldosteronism: implications for diagnosis, pathogenesis, and treatment. Endocr. Rev. 39, 1057–1088 (2018).
Caroccia, B. et al. Macrolides blunt aldosterone biosynthesis: a proof-of-concept study in KCNJ5 mutated adenoma cells ex vivo. Hypertension 70, 1238–1242 (2017).
Conn, J. W. Presidential address. I. Painting background. II. Primary aldosteronism, a new clinical syndrome. J. Lab. Clin. Med. 45, 3–17 (1955).
Conn, J. W. & Louis, L. H. Primary aldosteronism: a new clinical entity. Trans. Assoc. Am. Physicians 68, 215–231 (1955).
Gordon, R. D. et al. Clinical and pathological diversity of primary aldosteronism, including a new familial variety. Clin. Exp. Pharmacol. Physiol. 18, 283–286 (1991).
Funder, J. W. et al. Case detection, diagnosis, and treatment of patients with primary aldosteronism: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 93, 3266–3281 (2008).
Acknowledgements
The laboratory of M.-C.Z. is supported through institutional funding from INSERM, by the Agence Nationale pour la Recherche (ANR-15-CE14-0017-03, ANR-18-CE93-0003-01), the Fondation pour la Recherche Médicale (EQU201903007864) and by the H2020 project ENSAT-HT grant number 633983.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Autonomous aldosterone production
-
Aldosterone production that is autonomous from its physiological regulators and inappropriate to the salt and blood volume status of the individual.
- Bilateral adrenal hyperplasia
-
One of the two major causes of primary aldosteronism, also called idiopathic hyperaldosteronism, in which aldosterone is produced autonomously from both adrenal glands.
- Essential hypertension
-
Arterial hypertension without a known identifiable cause, defined after having excluded all forms secondary to a defined disease; it represents 85–95% of cases.
- Aldosterone lateralization indices
-
Values reflecting the production of aldosterone from one adrenal gland compared with the other, calculated by dividing the aldosterone to cortisol ratio in the dominant adrenal vein by the ratio in the non-dominant adrenal vein, measured by adrenal vein sampling.
- Lateralized aldosterone production
-
Autonomous aldosterone production from one adrenal gland.
- Lateralized primary aldosteronism
-
Primary aldosteronism due to autonomous aldosterone production from one adrenal gland.
Rights and permissions
About this article
Cite this article
Zennaro, MC., Boulkroun, S. & Fernandes-Rosa, F.L. Pathogenesis and treatment of primary aldosteronism. Nat Rev Endocrinol 16, 578–589 (2020). https://doi.org/10.1038/s41574-020-0382-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41574-020-0382-4
This article is cited by
-
Preoperative supine time for adrenal venous sampling: a prospective randomized controlled trial
Trials (2024)
-
The effect of different treatment strategies on glycolipid metabolism disorders and cardiovascular events in primary aldosteronism
Hypertension Research (2024)
-
A controlled trial of percutaneous adrenal arterial embolization for hypertension in patients with idiopathic hyperaldosteronism
Hypertension Research (2024)
-
Nanotechnology-based Detection Strategies for Hypertension Biomarkers
Chemical Research in Chinese Universities (2024)
-
The ex vivo perfused mouse adrenal gland—a new model to study aldosterone secretion
Pflügers Archiv - European Journal of Physiology (2024)