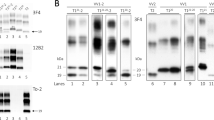
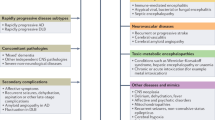
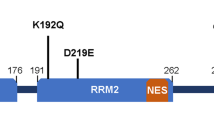
Abstract
Prion diseases share common clinical and pathological characteristics such as spongiform neuronal degeneration and deposition of an abnormal form of a host-derived protein, termed prion protein. The characteristic features of prion diseases are long incubation times, short clinical courses, extreme resistance of the transmissible agent to degradation and lack of nucleic acid involvement. Sporadic and genetic forms of prion diseases occur worldwide, of which genetic forms are associated with mutations in PRNP. Human to human transmission of these diseases has occurred due to iatrogenic exposure, and zoonotic forms of prion diseases are linked to bovine disease. Significant progress has been made in the diagnosis of these disorders. Clinical tools for diagnosis comprise brain imaging and cerebrospinal fluid tests. Aggregation assays for detection of the abnormally folded prion protein have a clear potential to diagnose the disease in peripherally accessible biofluids. After decades of therapeutic nihilism, new treatment strategies and clinical trials are on the horizon. Although prion diseases are relatively rare disorders, understanding their pathogenesis and mechanisms of prion protein misfolding has significantly enhanced the field in research of neurodegenerative diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 1 digital issues and online access to articles
$99.00 per year
only $99.00 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Alemà, G. in Proceedings of the 5th World Congress of Psychiatry (eds de la Fuente, R. & Weisman, M. N.) 1221–1227 (American Elsevier, 1973).
Masters, C. L. et al. Creutzfeldt-Jakob disease: patterns of worldwide occurrence and the significance of familial and sporadic clustering. Ann. Neurol. 5, 177–188 (1979).
Will, R. G. et al. Descriptive epidemiology of Creutzfeldt-Jakob disease in six European countries, 1993-1995. EU Collaborative Study Group for CJD. Ann. Neurol. 43, 763–767 (1998).
Watson, N. et al. The importance of ongoing international surveillance for Creutzfeldt-Jakob disease. Nat. Rev. Neurol. 17, 362–379 (2021).
Watson, N. et al. Validation of revised International Creutzfeldt-Jakob disease surveillance network diagnostic criteria for sporadic Creutzfeldt-Jakob disease. JAMA Netw. Open. 5, e2146319 (2022).
Ladogana, A. et al. Mortality from Creutzfeldt-Jakob disease and related disorders in Europe, Australia, and Canada. Neurology 64, 1586–1591 (2005). This study provides comparative data on disease mortality in Europe as a joint effort of national surveillance systems.
Ladogana, A. et al. Creutzfeldt-Jakob disease: the public health perception. Eur. J. Neurodeg Dis. 1, 101–113 (2012).
D’Aignaux, J. H. et al. Analysis of the geographical distribution of sporadic Creutzfeldt-Jakob disease in France between 1992 and 1998. Int. J. Epidemiol. 31, 490–495 (2002).
Nakatani, E. et al. Temporal and regional variations in sporadic Creutzfeldt-Jakob disease in Japan, 2001-2010. Epidemiol. Infect. 143, 1073–1078 (2015).
Puopolo, M. et al. Spatial epidemiology of sporadic Creutzfeldt-Jakob disease in Apulia, Italy. Neuroepidemiology 54, 83–90 (2020).
Klug, G. M. et al. Intensity of human prion disease surveillance predicts observed disease incidence. J. Neurol. Neurosurg. Psychiatry 84, 1372–1377 (2013).
Sun, Y. et al. Incidence of and mortality due to human prion diseases in Taiwan: a prospective 20-year nationwide surveillance study from 1998 to 2017. Clin. Epidemiol. 12, 1073–1081 (2020).
Stehmann, C. et al. Communicable Diseases Intelligence. Creutzfeldt-Jakob disease surveillance in Australia: update to 31 December 2022. Commonwealth of Australia Department of Health and Aged Care https://doi.org/10.33321/cdi.2023.47.37 (2023).
Kim, Y. C. & Jeong, B. H. Creutzfeldt-Jakob disease incidence, South Korea, 2001-2019. Emerg. Infect. Dis. 28, 1863–1866 (2022).
Government of Canada. Creutzfeldt-Jakob Disease Surveillance System (CJDSS) report. Government of Canada www.canada.ca/en/public-health/services/surveillance/blood-safety-contribution-program/creutzfeldt-jakob-disease/cjd-surveillance-system.html (2023).
Hermann, P. et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 20, 235–246 (2021). This paper summarizes the current knowledge on clinical biomarkers and consensus guidelines by experts from national CJD surveillance systems for clinical diagnosis.
Parchi, P. et al. Classification of sporadic Creutzfeldt-Jakob disease based on molecular and phenotypic analysis of 300 subjects. Ann. Neurol. 46, 224–233 (1999).
Denouel, A. et al. The role of environmental factors on sporadic Creutzfeldt-Jakob disease mortality: evidence from an age-period-cohort analysis. Eur. J. Epidemiol. 38, 757–764 (2023).
Pocchiari, M. et al. Predictors of survival in sporadic Creutzfeldt-Jakob disease and other human transmissible spongiform encephalopathies. Brain 127, 2348–2359 (2004).
Alperovitch, A. et al. Codon 129 prion protein genotype and sporadic Creutzfeldt-Jakob disease. Lancet 353, 1673–1674 (1999). This paper provides evidence for the role of the codon 129 genotype of the prion protein gene in disease susceptibility in sporadic CJD.
Palmer, M. S., Dryden, A. J., Hughes, J. T. & Collinge, J. Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 352, 340–342 (1991).
Prusiner, S. B. Prions. Proc. Natl Acad. Sci. USA 95, 13363–13383 (1998).
Mead, S., Lloyd, S. & Collinge, J. Genetic factors in mammalian prion diseases. Annu. Rev. Genet. 53, 117–147 (2019).
Brown, P. et al. Human spongiform encephalopathy: the National Institutes of Health series of 300 cases of experimentally transmitted disease. Ann. Neurol. 35, 513–529 (1994).
Tateishi, J. et al. First experimental transmission of fatal familial insomnia. Nature 376, 434–435 (1995).
Tateishi, J., Kitamoto, T., Hoque, M. Z. & Furukawa, H. Experimental transmission of Creutzfeldt-Jakob disease and related diseases to rodents. Neurology 46, 532–537 (1996).
Minikel, E. V. et al. Quantifying prion disease penetrance using large population control cohorts. Sci. Transl. Med. 8, 322ra329 (2016).
Kovacs, G. G. et al. Genetic prion disease: the EUROCJD experience. Hum. Genet. 118, 166–174 (2005).
Kim, D. Y., Shim, K. H., Bagyinszky, E. & An, S. S. A. Prion mutations in Republic of Republic of Korea, China, and Japan. Int. J. Mol. Sci. 24, 625 (2022).
Ladogana, A. & Kovacs, G. G. Genetic Creutzfeldt-Jakob disease. Handb. Clin. Neurol. 153, 219–242 (2018).
Brown, P. et al. Familial Creutzfeldt-Jakob disease in Chile is associated with the codon 200 mutation of the PRNP amyloid precursor gene on chromosome 20. J. Neurol. Sci. 112, 65–67 (1992).
Duffy, P. et al. Letter: possible person-to-person transmission of Creutzfeldt-Jakob disease. N. Engl. J. Med. 290, 692–693 (1974).
Brown, P. et al. Iatrogenic Creutzfeldt-Jakob disease, final assessment. Emerg. Infect. Dis. 18, 901–907 (2012).
Rudge, P. et al. Iatrogenic CJD due to pituitary-derived growth hormone with genetically determined incubation times of up to 40 years. Brain 138, 3386–3399 (2015).
National CJD. Surveillance Unit. Creutzfeldt-Jakob disease in the UK. NCJDRSU www.cjd.ed.ac.uk/sites/default/files/figs.pdf (2024).
Takumi, I. & Akino, K. Creutzfeldt-Jakob disease and lyodura:a special reference to prion disease control in the field of neurosurgery [Japanese]. No Shinkei Geka 50, 1078–1086 (2022).
Hamaguchi, T. et al. Insight into the frequent occurrence of dura mater graft-associated Creutzfeldt-Jakob disease in Japan. J. Neurol. Neurosurg. Psychiatry 84, 1171–1175 (2013).
Kobayashi, Y., Kitamoto, T. & Mizusawa, H. Iatrogenic Creutzfeldt-Jakob disease. Handb. Clin. Neurol. 153, 207–218 (2018).
Brandel, J. P. et al. Distribution of codon 129 genotype in human growth hormone-treated CJD patients in France and the UK. Lancet 362, 128–130 (2003).
Peckeu, L. et al. Factors influencing the incubation of an infectious form of Creutzfeldt-Jakob disease. Clin. Infect. Dis. 70, 1487–1490 (2020).
Will, R. G. et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet 347, 921–925 (1996).
Diack, A. B. et al. Variant CJD. 18 years of research and surveillance. Prion 8, 286–295 (2014).
Brandel, J. P. et al. Variant Creutzfeldt-Jakob disease diagnosed 7.5 years after occupational exposure. N. Engl. J. Med. 383, 83–85 (2020).
Santé publique France. Maladie de Creutzfeldt-Jakob [French]. Santé publique France www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-infectieuses-d-origine-alimentaire/maladie-de-creutzfeldt-jakob (2024).
Diack, A. B., Will, R. G. & Manson, J. C. Public health risks from subclinical variant CJD. PLoS Pathog. 13, e1006642 (2017).
Collinge, J. et al. Kuru in the 21st century – an acquired human prion disease with very long incubation periods. Lancet 367, 2068–2074 (2006).
Alpers, M. P. Review. The epidemiology of kuru: monitoring the epidemic from its peak to its end. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3707–3713 (2008).
Collinge, J. Mammalian prions and their wider relevance in neurodegenerative diseases. Nature 539, 217–226 (2016).
Kara, E., Marks, J. D. & Aguzzi, A. Toxic protein spread in neurodegeneration: reality versus fantasy. Trends Mol. Med. 24, 1007–1020 (2018).
Peng, C., Trojanowski, J. Q. & Lee, V. M. Protein transmission in neurodegenerative disease. Nat. Rev. Neurol. 16, 199–212 (2020).
Li, J., Browning, S., Mahal, S. P., Oelschlegel, A. M. & Weissmann, C. Darwinian evolution of prions in cell culture. Science 327, 869–872 (2010).
Marrero-Winkens, C. et al. From seeds to fibrils and back: fragmentation as an overlooked step in the propagation of prions and prion-like proteins. Biomolecules 10, 1305 (2020).
Orgel, L. E. Prion replication and secondary nucleation. Chem. Biol. 3, 413–414 (1996).
Bueler, H. et al. Mice devoid of PrP are resistant to scrapie. Cell 73, 1339–1347 (1993).
Benestad, S. L., Austbo, L., Tranulis, M. A., Espenes, A. & Olsaker, I. Healthy goats naturally devoid of prion protein. Vet. Res. 43, 87 (2012).
Richt, J. A. et al. Production of cattle lacking prion protein. Nat. Biotechnol. 25, 132–138 (2007).
Bueler, H. et al. High prion and PrPSc levels but delayed onset of disease in scrapie-inoculated mice heterozygous for a disrupted PrP gene. Mol. Med. 1, 19–30 (1994).
Mallucci, G. et al. Depleting neuronal PrP in prion infection prevents disease and reverses spongiosis. Science 302, 871–874 (2003). This paper underscores the causal involvement of the prion protein in prion infection and provides evidence for targeting PrP for therapy.
Minikel, E. V. et al. Prion protein lowering is a disease-modifying therapy across prion disease stages, strains and endpoints. Nucleic Acids Res. 48, 10615–10631 (2020).
Houston, F. & Andreoletti, O. Animal prion diseases: the risks to human health. Brain Pathol. 29, 248–262 (2019).
Sandberg, M. K. et al. Prion neuropathology follows the accumulation of alternate prion protein isoforms after infective titre has peaked. Nat. Commun. 5, 4347 (2014).
Artikis, E., Kraus, A. & Caughey, B. Structural biology of ex vivo mammalian prions. J. Biol. Chem. 298, 102181 (2022).
Riek, R. et al. NMR structure of the mouse prion protein domain PrP(121-231). Nature 382, 180–182 (1996).
Brown, D. R. et al. The cellular prion protein binds copper in vivo. Nature 390, 684–687 (1997).
Pan, K. M. et al. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc. Natl Acad. Sci. USA 90, 10962–10966 (1993).
Kraus, A. et al. High-resolution structure and strain comparison of infectious mammalian prions. Mol. Cell 81, 4540–4551.e6 (2021).
Manka, S. W. et al. 2.7 Å cryo-EM structure of ex vivo RML prion fibrils. Nat. Commun. 13, 4004 (2022).
Hoyt, F. et al. Cryo-EM structure of anchorless RML prion reveals variations in shared motifs between distinct strains. Nat. Commun. 13, 4005 (2022).
Hallinan, G. I. et al. Cryo-EM structures of prion protein filaments from Gerstmann–Sträussler–Scheinker disease. Acta Neuropathol. 144, 509–520 (2022).
Taylor, D. R. & Hooper, N. M. The prion protein and lipid rafts. Mol. Membr. Biol. 23, 89–99 (2006).
Linsenmeier, L. et al. Diverse functions of the prion protein – does proteolytic processing hold the key? Biochim. Biophys. Acta Mol. Cell Res. 1864, 2128–2137 (2017).
Skedsmo, F. S. et al. Demyelinating polyneuropathy in goats lacking prion protein. FASEB J. 34, 2359–2375 (2020).
Bremer, J. et al. Axonal prion protein is required for peripheral myelin maintenance. Nat. Neurosci. 13, 310–318 (2010).
Collinge, J. Variant Creutzfeldt-Jakob disease. Lancet 354, 317–323 (1999).
Bishop, M. T., Will, R. G. & Manson, J. C. Defining sporadic Creutzfeldt-Jakob disease strains and their transmission properties. Proc. Natl Acad. Sci. USA 107, 12005–12010 (2010).
Cassard, H. et al. Prions from sporadic Creutzfeldt-Jakob disease patients propagate as strain mixtures. mBio 11, e00393-20 (2020).
Bruce, M. et al. Transmission of bovine spongiform encephalopathy and scrapie to mice: strain variation and the species barrier. Philos. Trans. R. Soc. Lond. B Biol. Sci. 343, 405–411 (1994).
Mead, S. et al. Genetic risk factors for variant Creutzfeldt-Jakob disease: a genome-wide association study. Lancet Neurol. 8, 57–66 (2009).
Manka, S. W. et al. A structural basis for prion strain diversity. Nat. Chem. Biol. 19, 607–613 (2023).
Noble, G. P., Walsh, D. J., Miller, M. B., Jackson, W. S. & Supattapone, S. Requirements for mutant and wild-type prion protein misfolding in vitro. Biochemistry 54, 1180–1187 (2015).
Jones, E. et al. Identification of novel risk loci and causal insights for sporadic Creutzfeldt-Jakob disease: a genome-wide association study. Lancet Neurol. 19, 840–848 (2020).
Supattapone, S. Cofactor molecules: essential partners for infectious prions. Prog. Mol. Biol. Transl. Sci. 175, 53–75 (2020).
Legname, G. et al. Synthetic mammalian prions. Science 305, 673–676 (2004).
Aguilar-Calvo, P. et al. Shortening heparan sulfate chains prolongs survival and reduces parenchymal plaques in prion disease caused by mobile, ADAM10-cleaved prions. Acta Neuropathol. 139, 527–546 (2020).
Heikenwalder, M. et al. Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 307, 1107–1110 (2005).
Beringue, V. et al. Facilitated cross-species transmission of prions in extraneural tissue. Science 335, 472–475 (2012).
Sigurdson, C. J., Bartz, J. C. & Glatzel, M. Cellular and molecular mechanisms of prion disease. Annu. Rev. Pathol. 14, 497–516 (2019).
Fraser, H. Neuronal spread of scrapie agent and targeting of lesions within the retino-tectal pathway. Nature 295, 149–150 (1982).
Lakkaraju, A. K. K. et al. Glial activation in prion diseases is selectively triggered by neuronal PrPSc. Brain Pathol. 32, e13056 (2022).
Prinz, M. et al. Intrinsic resistance of oligodendrocytes to prion infection. J. Neurosci. 24, 5974–5981 (2004).
Bradford, B. M., McGuire, L. I., Hume, D. A., Pridans, C. & Mabbott, N. A. Microglia deficiency accelerates prion disease but does not enhance prion accumulation in the brain. Glia 70, 2169–2187 (2022).
Falsig, J. et al. A versatile prion replication assay in organotypic brain slices. Nat. Neurosci. 11, 109–117 (2008).
Scheckel, C., Imeri, M., Schwarz, P. & Aguzzi, A. Ribosomal profiling during prion disease uncovers progressive translational derangement in glia but not in neurons. Elife 9, e62911 (2020).
Sandberg, M. K., Al-Doujaily, H., Sharps, B., Clarke, A. R. & Collinge, J. Prion propagation and toxicity in vivo occur in two distinct mechanistic phases. Nature 470, 540–542 (2011).
Eskandari-Sedighi, G. et al. Quaternary structure changes for PrPSc predate PrPC downregulation and neuronal death during progression of experimental scrapie disease. Mol. Neurobiol. 58, 375–390 (2021).
Mok, T. H. et al. Seed amplification and neurodegeneration marker trajectories in individuals at risk of prion disease. Brain 146, 2570–2583 (2023).
Schmitz, M. et al. Detection of prion protein seeding activity in tear fluids. N. Engl. J. Med. 388, 1816–1817 (2023).
Vallabh, S. M. et al. Cerebrospinal fluid and plasma biomarkers in individuals at risk for genetic prion disease. BMC Med. 18, 140 (2020).
Mercer, R. C. C. & Harris, D. A. Mechanisms of prion-induced toxicity. Cell Tissue Res. 392, 81–96 (2023).
Brandner, S. et al. Normal host prion protein necessary for scrapie-induced neurotoxicity. Nature 379, 339–343 (1996).
Benilova, I. & De Strooper, B. Prion protein in Alzheimer’s pathogenesis: a hot and controversial issue. EMBO Mol. Med. 2, 289–290 (2010).
Collinge, J. Molecular neurology of prion disease. J. Neurol. Neurosurg. Psychiatry 76, 906–919 (2005).
Mallucci, G. R. et al. Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53, 325–335 (2007).
Chesebro, B. et al. Anchorless prion protein results in infectious amyloid disease without clinical scrapie. Science 308, 1435–1439 (2005).
Lauren, J., Gimbel, D. A., Nygaard, H. B., Gilbert, J. W. & Strittmatter, S. M. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-β oligomers. Nature 457, 1128–1132 (2009).
Balducci, C. & Forloni, G. Doxycycline for Alzheimer’s disease: fighting β-amyloid oligomers and neuroinflammation. Front. Pharmacol. 10, 738 (2019).
Fang, C. et al. Prions activate a p38 MAPK synaptotoxic signaling pathway. PLoS Pathog. 14, e1007283 (2018).
Moreno, J. A. et al. Sustained translational repression by eIF2ɑ-P mediates prion neurodegeneration. Nature 485, 507–511 (2012).
Lakkaraju, A. K. K. et al. Loss of PIKfyve drives the spongiform degeneration in prion diseases. EMBO Mol. Med. 13, e14714 (2021).
Smith, H. L. et al. Astrocyte unfolded protein response induces a specific reactivity state that causes non-cell-autonomous neuronal degeneration. Neuron 105, 855–866.e5 (2020).
Mallucci, G. R. et al. Post-natal knockout of prion protein alters hippocampal CA1 properties, but does not result in neurodegeneration. EMBO J. 21, 202–210 (2002).
Rhoads, D. D. et al. Diagnosis of prion diseases by RT-QuIC results in improved surveillance. Neurology 95, e1017–e1026 (2020).
World Health Organization. Global surveillance, diagnosis and therapy of human transmissible spongiform encephalopatheis: Report of WHO Consultation 1998. WHO iris.who.int/bitstream/handle/10665/65516/WHO_EMC_ZDI_98.9.pdf?sequence=1&isAllowed=y (1998).
Parchi, P. et al. Consensus classification of human prion disease histotypes allows reliable identification of molecular subtypes: an inter-rater study among surveillance centres in Europe and USA. Acta Neuropathol. 124, 517–529 (2012). This paper provides a clinical and neuropathological classification system for sporadic CJD based on molecular characteristics of the abnormal prion protein and codon 129 genotype of PRNP.
Zerr, I. & Parchi, P. Sporadic Creutzfeldt-Jakob disease. Handb. Clin. Neurol. 153, 155–174 (2018).
Mead, S. & Rudge, P. CJD mimics and chameleons. Pract. Neurol. 17, 113–121 (2017).
Hermann, P. & Zerr, I. Rapidly progressive dementias – aetiologies, diagnosis and management. Nat. Rev. Neurol. 18, 363–376 (2022).
Zerr, I. et al. Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132, 2659–2668 (2009).
Steinhoff, B. J. et al. Diagnostic value of periodic complexes in Creutzfeldt-Jakob disease. Ann. Neurol. 56, 702–708 (2004).
Muayqil, T., Gronseth, G. & Camicioli, R. Evidence-based guideline: diagnostic accuracy of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 79, 1499–1506 (2012).
Skillback, T. et al. Diagnostic performance of cerebrospinal fluid total tau and phosphorylated tau in Creutzfeldt-Jakob disease: results from the Swedish Mortality Registry. JAMA Neurol. 71, 476–483 (2014).
Stoeck, K. et al. Cerebrospinal fluid biomarker supported diagnosis of Creutzfeldt-Jakob disease and rapid dementias: a longitudinal multicentre study over 10 years. Brain 135, 3051–3061 (2012).
Lattanzio, F. et al. Prion-specific and surrogate CSF biomarkers in Creutzfeldt-Jakob disease: diagnostic accuracy in relation to molecular subtypes and analysis of neuropathological correlates of p-tau and Aβ42 levels. Acta Neuropathol. 133, 559–578 (2017).
Atarashi, R. et al. Ultrasensitive human prion detection in cerebrospinal fluid by real-time quaking-induced conversion. Nat. Med. 17, 175–178 (2011).
Orru, C. D. et al. Rapid and sensitive RT-QuIC detection of human Creutzfeldt-Jakob disease using cerebrospinal fluid. mBio 6, e02451-14 (2015). Together with Atarashi et al. (2011), this paper reports the new technology, RT-QuIC, for detection of misfolded PrP aggregates in biological fluids.
Orru, C. D. et al. A test for Creutzfeldt-Jakob disease using nasal brushings. N. Engl. J. Med. 371, 519–529 (2014).
Orru, C. D. et al. Prion seeding activity and infectivity in skin samples from patients with sporadic Creutzfeldt-Jakob disease. Sci. Transl. Med. 9, eaam7785 (2017).
Hermann, P. et al. Application of real-time quaking-induced conversion in Creutzfeldt-Jakob disease surveillance. J. Neurol. 270, 2149–2161 (2023).
Abu-Rumeileh, S. et al. Comparison between plasma and cerebrospinal fluid biomarkers for the early diagnosis and association with survival in prion disease. J. Neurol. Neurosurg. Psychiatry 91, 1181–1188 (2020).
Rudge, P., Hyare, H., Green, A., Collinge, J. & Mead, S. Imaging and CSF analyses effectively distinguish CJD from its mimics. J. Neurol. Neurosurg. Psychiatry 89, 461–466 (2018).
Bizzi, A. et al. Evaluation of a new criterion for detecting prion disease with diffusion magnetic resonance imaging. JAMA Neurol. 77, 1141–1149 (2020).
Yasuda, M. et al. Propagation of diffusion-weighted MRI abnormalities in the preclinical stage of sporadic Creutzfeldt-Jakob disease. Neurology 99, 699–702 (2022).
Abu-Rumeileh, S. et al. Sporadic fatal insomnia in Europe: phenotypic features and diagnostic challenges. Ann. Neurol. 84, 347–360 (2018).
Jesuthasan, A. et al. Assessing initial MRI reports for suspected CJD patients. J. Neurol. 269, 4452–4458 (2022).
Marquetand, J. et al. Periodic EEG patterns in sporadic Creutzfeld-Jakob-Disease can be benzodiazepine-responsive and be difficult to distinguish from non-convulsive status epilepticus. Seizure 53, 47–50 (2017).
Lapergue, B. et al. Sporadic Creutzfeldt-Jakob disease mimicking nonconvulsive status epilepticus. Neurology 74, 1995–1999 (2010).
Minikel, E. V. et al. Age at onset in genetic prion disease and the design of preventive clinical trials. Neurology 93, e125–e134 (2019).
Arata, H. et al. Early clinical signs and imaging findings in Gerstmann-Sträussler-Scheinker syndrome (Pro102Leu). Neurology 66, 1672–1678 (2006).
Krasnianski, A. et al. Fatal familial insomnia: clinical features and early identification. Ann. Neurol. 63, 658–661 (2008).
Schmitz, M. et al. Diagnostic accuracy of cerebrospinal fluid biomarkers in genetic prion diseases. Brain 145, 700–712 (2022).
Mead, S. et al. A novel prion disease associated with diarrhea and autonomic neuropathy. N. Engl. J. Med. 369, 1904–1914 (2013).
Llorens, F. et al. Diagnostic accuracy of prion disease biomarkers in iatrogenic Creutzfeldt-Jakob disease. Biomolecules 10, 290 (2020).
Heath, C. A. et al. Validation of diagnostic criteria for variant Creutzfeldt-Jakob disease. Ann. Neurol. 67, 761–770 (2010).
Moda, F. et al. Prions in the urine of patients with variant Creutzfeldt-Jakob disease. N. Engl. J. Med. 371, 530–539 (2014).
Lacroux, C. et al. Preclinical detection of variant CJD and BSE prions in blood. PLoS Pathog. 10, e1004202 (2014).
Mok, T. et al. Variant Creutzfeldt-Jakob disease in a patient with heterozygosity at PRNP codon 129. N. Engl. J. Med. 376, 292–294 (2017).
Paterson, R. W. et al. Differential diagnosis of Jakob-Creutzfeldt disease. Arch. Neurol. 69, 1578–1582 (2012).
Chitravas, N. et al. Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann. Neurol. 70, 437–444 (2011).
Maat, P. et al. Pathologically confirmed autoimmune encephalitis in suspected Creutzfeldt-Jakob disease. Neurol. Neuroimmunol. Neuroinflamm 2, e178 (2015).
Biggi, S. et al. Identification of compounds inhibiting prion replication and toxicity by removing PrPC from the cell surface. J. Neurochem. 152, 136–150 (2020).
Forloni, G., Roiter, I. & Tagliavini, F. Clinical trials of prion disease therapeutics. Curr. Opin. Pharmacol. 44, 53–60 (2019).
Baiardi, S., Mammana, A., Capellari, S. & Parchi, P. Human prion disease: molecular pathogenesis, and possible therapeutic targets and strategies. Expert Opin. Ther. Targets 27, 1271–1284 (2023).
Otto, M. et al. Efficacy of flupirtine on cognitive function in patients with CJD: a double-blind study. Neurology 62, 714–718 (2004).
Doh-Ura, K., Iwaki, T. & Caughey, B. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J. Virol. 74, 4894–4897 (2000).
Collins, S. J. et al. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann. Neurol. 52, 503–506 (2002).
Barret, A. et al. Evaluation of quinacrine treatment for prion diseases. J. Virol. 77, 8462–8469 (2003).
Murakami-Kubo, I. et al. Quinoline derivatives are therapeutic candidates for transmissible spongiform encephalopathies. J. Virol. 78, 1281–1288 (2004).
Haik, S. et al. Compassionate use of quinacrine in Creutzfeldt-Jakob disease fails to show significant effects. Neurology 63, 2413–2415 (2004).
Nakajima, M. et al. Results of quinacrine administration to patients with Creutzfeldt-Jakob disease. Dement. Geriatr. Cogn. Disord. 17, 158–163 (2004).
Collinge, J. et al. Safety and efficacy of quinacrine in human prion disease (PRION-1 study): a patient-preference trial. Lancet Neurol. 8, 334–344 (2009).
Geschwind, M. D. et al. Quinacrine treatment trial for sporadic Creutzfeldt-Jakob disease. Neurology 81, 2015–2023 (2013).
Ghaemmaghami, S. et al. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 5, e1000673 (2009).
Schenk, D. et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400, 173–177 (1999).
van Dyck, C. H. et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21 (2023).
Sims, J. R. et al. Donanemab in early symptomatic Alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA 330, 512–527 (2023).
Gabizon, R., McKinley, M. P., Groth, D. & Prusiner, S. B. Immunoaffinity purification and neutralization of scrapie prion infectivity. Proc. Natl Acad. Sci. USA 85, 6617–6621 (1988).
Enari, M., Flechsig, E. & Weissmann, C. Scrapie prion protein accumulation by scrapie-infected neuroblastoma cells abrogated by exposure to a prion protein antibody. Proc. Natl Acad. Sci. USA 98, 9295–9299 (2001). This study provides evidence for targeting PrP for therapy.
Peretz, D. et al. Antibodies inhibit prion propagation and clear cell cultures of prion infectivity. Nature 412, 739–743 (2001).
Fernandez-Borges, N. et al. DNA vaccination can break immunological tolerance to PrP in wild-type mice and attenuates prion disease after intracerebral challenge. J. Virol. 80, 9970–9976 (2006).
Nitschke, C. et al. Immunisation strategies against prion diseases: prime-boost immunisation with a PrP DNA vaccine containing foreign helper T-cell epitopes does not prevent mouse scrapie. Vet. Microbiol. 123, 367–376 (2007).
Solforosi, L. et al. Cross-linking cellular prion protein triggers neuronal apoptosis in vivo. Science 303, 1514–1516 (2004).
Sonati, T. et al. The toxicity of antiprion antibodies is mediated by the flexible tail of the prion protein. Nature 501, 102–106 (2013).
Frontzek, K. et al. A conformational switch controlling the toxicity of the prion protein. Nat. Struct. Mol. Biol. 29, 831–840 (2022).
Heppner, F. L. et al. Prevention of scrapie pathogenesis by transgenic expression of anti-prion protein antibodies. Science 294, 178–182 (2001).
White, M. D. et al. Single treatment with RNAi against prion protein rescues early neuronal dysfunction and prolongs survival in mice with prion disease. Proc. Natl Acad. Sci. USA 105, 10238–10243 (2008).
Reimann, R. R. et al. Differential toxicity of antibodies to the prion protein. PLoS Pathog. 12, e1005401 (2016).
Mead, S. et al. Prion protein monoclonal antibody (PRN100) therapy for Creutzfeldt-Jakob disease: evaluation of a first-in-human treatment programme. Lancet Neurol. 21, 342–354 (2022). This paper reports on successful administration and clinical effect of an anti-prion protein antibody.
Tagliavini, F. et al. Tetracycline affects abnormal properties of synthetic PrP peptides and PrPSc in vitro. J. Mol. Biol. 300, 1309–1322 (2000).
Forloni, G. et al. Tetracyclines affect prion infectivity. Proc. Natl Acad. Sci. USA 99, 10849–10854 (2002). This paper provides a rationale for treatment with doxycycline.
De Luigi, A. et al. The efficacy of tetracyclines in peripheral and intracerebral prion infection. PLoS ONE 3, e1888 (2008).
Lucchetti, J. et al. Plasma and brain concentrations of doxycycline after single and repeated doses in wild-type and APP23 mice. J. Pharmacol. Exp. Ther. 368, 32–40 (2019).
Forloni, G., Salmona, M., Marcon, G. & Tagliavini, F. Tetracyclines and prion infectivity. Infect. Disord. Drug. Targets 9, 23–30 (2009).
Haik, S. et al. Doxycycline in Creutzfeldt-Jakob disease: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 13, 150–158 (2014).
Varges, D. et al. Doxycycline in early CJD: a double-blinded randomised phase II and observational study. J. Neurol. Neurosurg. Psychiatry 88, 119–125 (2017).
Hannaoui, S. et al. Cycline efficacy on the propagation of human prions in primary cultured neurons is strain-specific. J. Infect. Dis. 209, 1144–1148 (2014).
Forloni, G. et al. Preventive study in subjects at risk of fatal familial insomnia: innovative approach to rare diseases. Prion 9, 75–79 (2015).
Forloni, G. et al. Preventive pharmacological treatment in subjects at risk for fatal familial insomnia: science and public engagement. Prion 16, 66–77 (2022).
Zomosa-Signoret, V., Arnaud, J. D., Fontes, P., Alvarez-Martinez, M. T. & Liautard, J. P. Physiological role of the cellular prion protein. Vet. Res. 39, 9 (2008).
Nazor Friberg, K. et al. Intracerebral infusion of antisense oligonucleotides into prion-infected mice. Mol. Ther. Nucleic Acids 1, e9 (2012).
Ahn, M. et al. Convection-enhanced delivery of AAV2-PrPshRNA in prion-infected mice. PLoS ONE 9, e98496 (2014).
Masone, A. et al. A tetracationic porphyrin with dual anti-prion activity. iScience 26, 107480 (2023).
Bennett, C. F., Krainer, A. R. & Cleveland, D. W. Antisense oligonucleotide therapies for neurodegenerative diseases. Annu. Rev. Neurosci. 42, 385–406 (2019).
Finkel, R. S. et al. Nusinersen versus sham control in infantile-onset spinal muscular atrophy. N. Engl. J. Med. 377, 1723–1732 (2017).
Miller, T. M. et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 387, 1099–1110 (2022).
Blair, H. A. Tofersen: first approval. Drugs 83, 1039–1043 (2023).
Kumar, D., Hasan, G. M., Islam, A. & Hassan, M. I. Therapeutic targeting of Huntington’s disease: molecular and clinical approaches. Biochem. Biophys. Res. Commun. 655, 18–24 (2023).
Raymond, G. J. et al. Antisense oligonucleotides extend survival of prion-infected mice. JCI Insight 5, e131175 (2019).
Vallabh, S. M., Minikel, E. V., Schreiber, S. L. & Lander, E. S. Towards a treatment for genetic prion disease: trials and biomarkers. Lancet Neurol. 19, 361–368 (2020).
Thompson, A. G. et al. The Medical Research Council prion disease rating scale: a new outcome measure for prion disease therapeutic trials developed and validated using systematic observational studies. Brain 136, 1116–1127 (2013).
Brandel, J. P., Welaratne, A., Denouel, A. & Haik, S. Validation of the Medical Research Council prion disease rating scale in France. Brain Commun. 5, fcad267 (2023).
Nihat, A. et al. Development of prognostic models for survival and care status in sporadic Creutzfeldt-Jakob disease. Brain Commun. 4, fcac201 (2022).
Appleby, B. S. & Yobs, D. R. Symptomatic treatment, care, and support of CJD patients. Handb. Clin. Neurol. 153, 399–408 (2018).
Harrison, K. L. et al. Developing neuropalliative care for sporadic Creutzfeldt-Jakob disease. Prion 16, 23–39 (2022).
McNiven, K. et al. Enteral feeding is associated with longer survival in the advanced stages of prion disease. Brain Commun. 1, fcz012 (2019).
Uflacker, A., Edmondson, M. C., Onyike, C. U. & Appleby, B. S. Caregiver burden in atypical dementias: comparing frontotemporal dementia, Creutzfeldt-Jakob disease, and Alzheimer’s disease. Int. Psychogeriatr. 28, 269–273 (2016).
Arpinelli, F. & Bamfi, F. The FDA guidance for industry on PROs: the point of view of a pharmaceutical company. Health Qual. Life Outcomes 4, 85 (2006).
Zerr, I. & Hermann, P. in Prion and Diseases (eds Zou, W. Q. & Gambetti, P.) 675–701 (Springer, 2023).
Nationales Referenzzentrum. CJD in Germany (11.12.2023). NRZ cjd-goettingen.de/en/news/cjd-figures/cjd-in-germany/ (2023).
Istituto Superiore di Sanità. Registers and surveillance: National Registry of Creutzfeldt-Jakob disease and related disorders. Istituto Superiore di Sanità www.iss.it/en/registro-mcj-dati-epidemiologici (2023).
UN Department of Economic and Social Affairs Statistics Division. Population and vital statistics report. UN unstats.un.org/unsd/demographic-social/products/vitstats/seratab2.pdf (2024).
Cashman, N. R. & Caughey, B. Prion diseases – close to effective therapy? Nat. Rev. Drug. Discov. 3, 874–884 (2004).
Author information
Authors and Affiliations
Contributions
Introduction (I.Z.); Epidemiology (A.L.); Mechanisms/pathophysiology (S.M.); Diagnosis, screening and prevention (P.H. and I.Z.); Management (G.F.); Quality of life (B.S.A.); Outlook (I.Z.); overview of Primer (I.Z.).
Corresponding author
Ethics declarations
Competing interests
I.Z. declares funding from BMG through the Robert Koch Institute, CJD Foundation and JPND; and consulting for IONIS, Sangamo, Gate Bio, Biogen and Lilly. B.S.A. declares funding from CDC, NIH, CJD Foundation, Ionis and Alector; consulting for Ionis, Sangamo, Gate Bio and Merck; and royalties from Wolters Kluwer. All other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Disease Primers thanks O. Andréoletti, H. Budka, G. Legname, P. Liberski and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zerr, I., Ladogana, A., Mead, S. et al. Creutzfeldt–Jakob disease and other prion diseases. Nat Rev Dis Primers 10, 14 (2024). https://doi.org/10.1038/s41572-024-00497-y
Accepted:
Published:
DOI: https://doi.org/10.1038/s41572-024-00497-y