Abstract
During evolution, humans developed innate and adaptive immune systems to survive infection by pathogens. The immune system needs to be finely balanced, as an overactive immune system can result in autoimmunity, allergy and inflammatory disorders, whereas over-attenuation can result in infections and cancer. Here, we describe how the immune system can be modulated using chemical approaches, with a focus on the chemical modification and application of antibodies, one of the major components of the immune defence system. Progress includes the development of antibody–drug conjugates and imaging reagents based on antibody fragments and nanobodies, and their clinical and preclinical applications for the treatment of cancer and autoimmune disease are discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Ehrlich, P. Beiträge zur Kenntniss der Anilinfärbungen und ihrer Verwendung in der mikroskopischen Technik. Arch. Mikrosk. Anat. 13, 263–277 (1877).
Ehrlich, P. Address in Pathology, ON CHEMIOTHERAPY: delivered before the Seventeenth International Congress of Medicine. Br. Med. J. 2, 353–359 (1913).
Bosch, F. & Rosich, L. The contributions of Paul Ehrlich to pharmacology: a tribute on the occasion of the centenary of his Nobel Prize. Pharmacology 82, 171–179 (2008).
Parascandola, J. The History of Salvarsan (Arsphenamine). eLS https://doi.org/10.1038/npg.els.0003622 (2001).
Medzhitov, R. Recognition of microorganisms and activation of the immune response. Nature 449, 819 (2007).
Akira, S. & Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 4, 499–511 (2004).
Casanova, J.-L. Adaptive immunity by convergent evolution. Nat. Rev. Immunol. 18, 294 (2018).
Cooper, M. D. & Alder, M. N. The evolution of adaptive immune systems. Cell 124, 815–822 (2006).
Krangel, M. S. Mechanics of T cell receptor gene rearrangement. Curr. Opin. Immunol. 21, 133–139 (2009).
Farber, D. L., Netea, M. G., Radbruch, A., Rajewsky, K. & Zinkernagel, R. M. Immunological memory: lessons from the past and a look to the future. Nat. Rev. Immunol. 16, 124 (2016).
Borel, J. F., Feurer, C., Gubler, H. U. & Stahelin, H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Act. 6, 468–475 (1976).
Rao, A., Luo, C. & Hogan, P. G. Transcription factors of the NFAT family: regulation and function. Annu. Rev. Immunol. 15, 707–747 (1997).
Calne, R. Y. et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 2, 1033–1036 (1979).
Kino, T. et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical and biological characteristics. J. Antbiot. 40, 1249–1255 (1987).
Kunz, J. & Hall, M. N. Cyclosporin A, FK506 and rapamycin: more than just immunosuppression. Trends Biochem. Sci. 18, 334–338 (1993).
Klintmalm, G. A review of FK506: a new immunosuppressant agent for the prevention and rescue of graft rejection. Transplant. Rev. 8, 53–63 (1994).
Ho, S. et al. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 80, S40–45 (1996).
Li, J., Kim, S. G. & Blenis, J. Rapamycin: one drug, many effects. Cell Metab. 19, 373–379 (2014).
Durnian, J. M., Stewart, R. M., Tatham, R., Batterbury, M. & Kaye, S. B. Cyclosporin-A associated malignancy. Clin. Ophthalmol. 1, 421–430 (2007).
Guba, M., Graeb, C., Jauch, K. W. & Geissler, E. K. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation 77, 1777–1782 (2004).
Sedger, L. M. & McDermott, M. F. TNF and TNF-receptors: From mediators of cell death and inflammation to therapeutic giants – past, present and future. Cytokine Growth Factor Rev. 25, 453–472 (2014).
Malottki, K. et al. Adalimumab, etanercept, infliximab, rituximab and abatacept for the treatment of rheumatoid arthritis after the failure of a tumour necrosis factor inhibitor: a systematic review and economic evaluation. Health Technol. Assess. 15, 1–278 (2011).
Bongartz, T. et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 295, 2275–2285 (2006).
Wilhelm, S. M., McKenney, K. A., Rivait, K. N. & Kale-Pradhan, P. B. A review of infliximab use in ulcerative colitis. Clin. Ther. 30, 223–230 (2008).
Han, C. et al. The impact of infliximab treatment on quality of life in patients with inflammatory rheumatic diseases. Arthritis Res. Ther. 9, R103 (2007).
Knight, D. M. et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol. Immunol. 30, 1443–1453 (1993).
Medler, T. R., Cotechini, T. & Coussens, L. M. Immune response to cancer therapy: mounting an effective antitumor response and mechanisms of resistance. Trends Cancer 1, 66–75 (2015).
Coley, W. B. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. 1893. Clin. Orthop. Relat. Res. 262, 3–11 (1991).
Rosenberg, S. A. Adoptive immunotherapy of cancer: accomplishments and prospects. Cancer Treat. Rep. 68, 233–255 (1984).
Baumeister, S. H., Freeman, G. J., Dranoff, G. & Sharpe, A. H. Coinhibitory pathways in immunotherapy for cancer. Annu. Rev. Immunol. 34, 539–573 (2016).
Sharpe, A. H. & Pauken, K. E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 18, 153–167 (2018).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98–106 (2016).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Callahan, M. K. & Wolchok, J. D. At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukocyte Biol. 94, 41–53 (2013).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Heinzerling, L. & Goldinger, S. M. A review of serious adverse effects under treatment with checkpoint inhibitors. Curr. Opin. Oncol. 29, 136–144 (2017).
Nossal, G. J. & Lederberg, J. Antibody production by single cells. Nature 181, 1419–1420 (1958).
Nutt, S. L., Hodgkin, P. D., Tarlinton, D. M. & Corcoran, L. M. The generation of antibody-secreting plasma cells. Nat. Rev. Immunol. 15, 160 (2015).
Ramaraj, T., Angel, T., Dratz, E. A., Jesaitis, A. J. & Mumey, B. Antigen–antibody interface properties: composition, residue interactions, and features of 53 non-redundant structures. Biochim. Biophys. Acta 1824, 520–532 (2012).
Friguet, B., Chaffotte, A. F., Djavadi-Ohaniance, L. & Goldberg, M. E. Measurements of the true affinity constant in solution of antigen-antibody complexes by enzyme-linked immunosorbent assay. J. Immunol. Methods 77, 305–319 (1985).
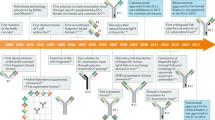
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Nadler, L. M. et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 40, 3147–3154 (1980).
McCafferty, J., Griffiths, A. D., Winter, G. & Chiswell, D. J. Phage antibodies: filamentous phage displaying antibody variable domains. Nature 348, 552–554 (1990).
Riechmann, L., Clark, M., Waldmann, H. & Winter, G. Reshaping human antibodies for therapy. Nature 332, 323–327 (1988).
Jones, P. T., Dear, P. H., Foote, J., Neuberger, M. S. & Winter, G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature 321, 522–525 (1986).
Teo, E. C., Chew, Y. & Phipps, C. A review of monoclonal antibody therapies in lymphoma. Crit. Rev. Oncol. Hematol. 97, 72–84 (2016).
Yan, L., Hsu, K. & Beckman, R. A. Antibody-based therapy for solid tumors. Cancer J. 14, 178–183 (2008).
Rosman, Z., Shoenfeld, Y. & Zandman-Goddard, G. Biologic therapy for autoimmune diseases: an update. BMC Med. 11, 88 (2013).
Jacob Sh,T. & Fletcher, T. E. Trial of ZMapp for Ebola virus infection. N. Engl. J. Med. 376, 700 (2017).
Agarwal, P. & Bertozzi, C. R. Site-specific antibody–drug conjugates: the nexus of bioorthogonal chemistry, protein engineering, and drug development. Bioconjug.Chem. 26, 176–192 (2014).
Irani, V. et al. Molecular properties of human IgG subclasses and their implications for designing therapeutic monoclonal antibodies against infectious diseases. Mol. Immunol. 67, 171–182 (2015).
Mantis, N. J., Rol, N. & Corthésy, B. Secretory IgA’s complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4, 603 (2011).
Simister, N. E. Placental transport of immunoglobulin G. Vaccine 21, 3365–3369 (2003).
Mankarious, S. et al. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 112, 634–640 (1988).
Liu, J. K. H. The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann. Med. Surg. 3, 113–116 (2014).
Fanale, M. A. & Younes, A. Monoclonal antibodies in the treatment of non-Hodgkin’s lymphoma. Drugs 67, 333–350 (2007).
Christian, B. A. & Lin, T. S. Antibody therapy for CLL. Semin. Hematol. 45, 95–103 (2008).
Martinelli, E., De Palma, R., Orditura, M., De Vita, F. & Ciardiello, F. Anti-epidermal growth factor receptor monoclonal antibodies in cancer therapy. Clin. Exp. Immunol. 158, 1–9 (2009).
Nakai, K., Hung, M.-C. & Yamaguchi, H. A perspective on anti-EGFR therapies targeting triple-negative breast cancer. Am. J. Cancer. Res. 6, 1609–1623 (2016).
Killock, D. Leveraging ADCC to enhance anti-HER2 therapy. Nat. Rev. Clin. Oncol. 14, 200 (2017).
Cameron, F., Whiteside, G. & Perry, C. Ipilimumab: first global approval. Drugs 71, 1093–1104 (2011).
Sun, C., Mezzadra, R. & Schumacher, T. N. Regulation and function of the PD-L1 checkpoint. Immunity 48, 434–452 (2018).
Seidel, J. A., Otsuka, A. & Kabashima, K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front. Oncol. 8, 86 (2018).
Evans, J. B. & Syed, B. A. Next-generation antibodies. Nat. Rev. Drug Discov. 13, 413–414 (2014).
Trail, P. A. et al. Cure of xenografted human carcinomas by BR96-doxorubicin immunoconjugates. Science 261, 212–215 (1993).
Anderl, J., Faulstich, H., Hechler, T. & Kulke, M. Antibody-drug conjugate payloads. Methods Mol. Biol. 1045, 51–70 (2013).
van der Lee, M. M. C. et al. The preclinical profile of the duocarmycin-based HER2-targeting ADC SYD985 predicts for clinical benefit in low HER2-expressing breast cancers. Mol. Cancer Ther. 14, 692–703 (2015).
Erickson, H. K. et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 66, 4426–4433 (2006).
Kellogg, B. A. et al. Disulfide-linked antibody-maytansinoid conjugates: optimization of in vivo activity by varying the steric hindrance at carbon atoms adjacent to the disulfide linkage. Bioconjug. Chem. 22, 717–727 (2011).
McDonagh, C. F. et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol. Cancer Ther. 11, 582–593 (2012).
Kontermann, R. E. & Brinkmann, U. Bispecific antibodies. Drug Discov. Today 20, 838–847 (2015).
de Goeij, B. E. C. G. et al. Efficient payload delivery by a bispecific antibody–drug conjugate targeting HER2 and CD63. Mol. Cancer Ther. 15, 2688–2697 (2016).
Mitsunaga, M. et al. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med 17, 1685–1691 (2011).
Ash, C., Dubec, M., Donne, K. & Bashford, T. Effect of wavelength and beam width on penetration in light-tissue interaction using computational methods. Lasers Med. Sci. 32, 1909–1918 (2017).
Matsumura, Y. Cancer stromal targeting (CAST) therapy. Adv. Drug Delivery Rev. 64, 710–719 (2012).
Polson, A. G. et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res. 69, 2358–2364 (2009).
Gébleux, R., Stringhini, M., Casanova, R., Soltermann, A. & Neri, D. Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Intl. J. Cancer 140, 1670–1679 (2017).
Rossin, R. et al. Chemically triggered drug release from an antibody-drug conjugate leads to potent antitumour activity in mice. Nat. Commun. 9, 1484 (2018).
Rossin, R. et al. Triggered drug release from an antibody-drug conjugate using fast “click-to-release” chemistry in mice. Bioconjug. Chem. 27, 1697–1706 (2016).
Holliger, P., Prospero, T. & Winter, G. “Diabodies”: small bivalent and bispecific antibody fragments. Proc. Natl Acad. Sci. USA 90, 6444–6448 (1993).
Huehls, A. M., Coupet, T. A. & Sentman, C. L. Bispecific T cell engagers for cancer immunotherapy. Immunol. Cell Biol. 93, 290–296 (2015).
Mullard, A. FDA approves first bispecific. Nat. Rev. Drug Discov. 14, 7 (2014).
Walseng, E. et al. Chemically programmed bispecific antibodies in diabody format. J. Biol. Chem. 291, 19661–19673 (2016).
Porter, R. R. The hydrolysis of rabbit γ-globulin and antibodies with crystalline papain. Biochem. J. 73, 119–126 (1959).
Nisonoff, A., Wissler, F. C., Lipman, L. N. & Woernley, D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch. Biochem. Biophys. 89, 230–244 (1960).
Parham, P., Androlewicz, M. J., Brodsky, F. M., Holmes, N. J. & Ways, J. P. Monoclonal antibodies: Purification, fragmentation and application to structural and functional studies of class I MHC antigens. J. Immunol. Methods 53, 133–173 (1982).
Huston, J. S. et al. Protein engineering of antibody binding sites: recovery of specific activity in an anti-digoxin single-chain Fv analogue produced in Escherichia coli. Proc. Natl Acad. Sci. USA 85, 5879–5883 (1988).
Nelson, A. L. Antibody fragments: hope and hype. mAbs 2, 77–83 (2010).
Testa, L. et al. Pexelizumab in ischemic heart disease: a systematic review and meta-analysis on 15,196 patients. J. Thorac. Cardiovasc. Surg. 136, 884–893 (2008).
Van Audenhove, I. & Gettemans, J. Nanobodies as versatile tools to understand, diagnose, visualize and treat cancer. eBioMedicine 8, 40–48 (2016).
Pleiner, T. et al. Nanobodies: site-specific labeling for super-resolution imaging, rapid epitope-mapping and native protein complex isolation. eLife 4, e11349 (2015).
Ta, D. T. et al. An efficient protocol towards site-specifically clickable nanobodies in high yield: cytoplasmic expression in Escherichia coli combined with intein-mediated protein ligation. Protein Eng. Des. Sel. 28, 351–363 (2015).
Rashidian, M. et al. Use of 18F-2-fluorodeoxyglucose to label antibody fragments for immuno-positron emission tomography of pancreatic cancer. ACS Cent. Sci. 1, 142–147 (2015).
Fang, T. et al. Structurally defined αMHC-II nanobody-drug conjugates: a therapeutic and imaging system for B-cell lymphoma. Angew. Chem. Int. Ed. Engl. 55, 2416–2420 (2016).
Rashidian, M. et al. Predicting the response to CTLA-4 blockade by longitudinal noninvasive monitoring of CD8 T cells. J. Exp. Med. 214, 2243–2255 (2017).
Vincke, C. et al. General strategy to humanize a camelid single-domain antibody and identification of a universal humanized nanobody scaffold. J. Biol. Chem. 284, 3273–3284 (2009).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02553317 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02287922 (2014).
Koide, A., Bailey, C. W., Huang, X. & Koide, S. The fibronectin type III domain as a scaffold for novel binding proteins. J. Mol. Biol. 284, 1141–1151 (1998).
Beste, G., Schmidt, F. S., Stibora, T. & Skerra, A. Small antibody-like proteins with prescribed ligand specificities derived from the lipocalin fold. Proc. Natl Acad. Sci. USA 96, 1898–1903 (1999).
Binz, H. K., Stumpp, M. T., Forrer, P., Amstutz, P. & Plückthun, A. Designing repeat proteins: well-expressed, soluble and stable proteins from combinatorial libraries of consensus ankyrin repeat proteins. J. Mol. Biol. 332, 489–503 (2003).
Nord, K. et al. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat. Biotechnol. 15, 772 (1997).
Nord, K., Nilsson, J., Nilsson, B., Uhlén, M. & Nygren, P.-Å. A combinatorial library of an α-helical bacterial receptor domain. Protein Eng. Des. Sel. 8, 601–608 (1995).
Lorey, S. et al. Novel ubiquitin-derived high affinity binding proteins with tumor targeting properties. J. Biol. Chem. 289, 8493–8507 (2014).
Leung, I., Jarvik, N. & Sidhu, S. S. A. Highly diverse and functional naïve ubiquitin variant library for generation of intracellular affinity reagents. J. Mol. Biol. 429, 115–127 (2017).
Li, D.-L. et al. Multifunctional superparamagnetic nanoparticles conjugated with fluorescein-labeled designed ankyrin repeat protein as an efficient HER2-targeted probe in breast cancer. Biomaterials 147, 86–98 (2017).
Ståhl, S. et al. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 35, 691–712 (2017).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03325621 (2017).
Mross, K. et al. First-in-human phase i study of PRS-050 (Angiocal), an anticalin targeting and antagonizing VEGF-A, in patients with advanced solid tumors. PLOS ONE 8, e83232 (2013).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03330561 (2017).
Kelley, J., de Bono, B. & Trowsdale, J. IRIS: A database surveying known human immune system genes. Genomics 85, 503–511 (2005).
Le Bourhis, L. et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLOS Pathog. 9, e1003681 (2013).
Oosenbrug, T., van de Graaff, M. J., Ressing, M. E. & van Kasteren, S. I. Chemical tools for studying TLR signaling dynamics. Cell Chem. Biol. 24, 801–812 (2017).
Mancini, R. J., Stutts, L., Ryu, K. A., Tom, J. K. & Esser-Kahn, A. P. Directing the immune system with chemical compounds. ACS Chem. Biol. 9, 1075–1085 (2014).
Khan, S. et al. Distinct uptake mechanisms but similar intracellular processing of two different toll-like receptor ligand-peptide conjugates in dendritic cells. J. Biol. Chem. 282, 21145–21159 (2007).
McDonald, D. M., Byrne, S. N. & Payne, R. J. Synthetic self-adjuvanting glycopeptide cancer vaccines. Front. Chem. 3, 60 (2015).
Hos, B. J., Tondini, E., van Kasteren, S. I. & Ossendorp, F. Approaches to improve chemically defined synthetic peptide vaccines. Front. Immunol. 9, 884 (2018).
Li, B. & Yang, R. Interaction between Yersinia pestis and the host immune system. Infect. Immun. 76, 1804–1811 (2008).
Gomery, K. et al. Antibody WN1 222–225 mimics Toll-like receptor 4 binding in the recognition of LPS. Proc. Natl Acad. Sci. USA 109, 20877–20882 (2012).
Li, J. & Chen, P. R. Development and application of bond cleavage reactions in bioorthogonal chemistry. Nat. Chem. Biol. 12, 129–137 (2016).
van der Gracht, A. M. F. et al. Chemical control over T-cell activation in vivo using deprotection of trans-cyclooctene-modified epitopes. ACS Chem. Biol. https://doi.org/10.1021/acschembio.8b00155 (2018).
Pawlak, J. B. et al. Bioorthogonal deprotection on the dendritic cell surface for chemical control of antigen cross-presentation. Angew. Chem. Int. Ed. Engl. 54, 5628–5631 (2015).
Ignacio, B. J., Albin, T., Esser-Kahn, A. P. & Verdoes, M. TLR agonist conjugation: a chemical perspective. Bioconjug. Chem. (2018).
Kummer, C., Petrich, B. G., Rose, D. M. & Ginsberg, M. H. A. Small molecule that inhibits the interaction of paxillin and α4 integrin inhibits accumulation of mononuclear leukocytes at a site of inflammation. J. Biol. Chem. 285, 9462–9469 (2010).
Fernandez, A. et al. Chemical modulation of in vivo macrophage function with subpopulation-specific fluorescent prodrug conjugates. ACS Cent. Sci. 3, 995–1005 (2017).
M., V. R. et al. Click-to-release from trans-cyclooctenes: mechanistic insights and expansion of scope from established carbamate to remarkable ether cleavage. Angew. Chem. Int. Ed. Engl. https://doi.org/10.1002/Anie.201800402 (2018).
Pictet, A. & Spengler, T. Über die bildung von isochinolin-derivaten durch einwirkung von methylal auf phenyl-äthylamin, phenyl-alanin und tyrosin. Ber. Dtsch. Chem. Ges 44, 2030–2036 (1911).
Ziegler, S. F. Division of labour by CD4+ T helper cells. Nat. Rev. Immunol. 16, 403 (2016).
Winau, F. & Winau, R. Emil von Behring and serum therapy. Microbes Infect. 4, 185–188 (2002).
Lindenmann, J. Origin of the terms ‘antibody’ and ‘antigen’. Scand. J. Immunol. 19, 281–285 (1984).
Burns, C. M. The history of cortisone discovery and development. Rheum. Dis. Clin. North Am. 42, 1–14 (2016).
Inbar, D., Hochman, J. & Givol, D. Localization of antibody-combining sites within the variable portions of heavy and light chains. Proc. Natl Acad. Sci. USA 69, 2659–2662 (1972).
Rosenberg, S. A. IL-2: the first effective immunotherapy for human cancer. J. Immunol. 192, 5451–5458 (2014).
Ortho Multicenter Transplant Study Group. A randomized clinical trial of OKT3 monoclonal antibody for acute rejection of cadaveric renal transplants. N. Engl. J. Med. 313, 337–342 (1985).
Monaco, C., Nanchahal, J., Taylor, P. & Feldmann, M. Anti-TNF therapy: past, present and future. Int. Immunol. 27, 55–62 (2015).
Diamantis, N. & Banerji, U. Antibody-drug conjugates — an emerging class of cancer treatment. Br. J. Cancer 114, 362–367 (2016).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Eggermont, A. M. & Robert, C. New drugs in melanoma: it’s a whole new world. Eur. J. Cancer 47, 2150–2157 (2011).
Arabi, F., Torabi-Rahvar, M., Shariati, A., Ahmadbeigi, N. & Naderi, M. Antigenic targets of CAR T cell therapy. A retrospective view on clinical trials. Exp. Cell Res. 369, 1–10 (2018).
Macian, F. NFAT proteins: key regulators of T cell development and function. Nat. Rev. Immunol. 5, 472 (2005).
Vincenti, F. et al. Belatacept and long-term outcomes in kidney transplantation. N. Engl. J. Med. 374, 333–343 (2016).
Acknowledgements
This work was supported by grants from the Gravity programme Institute of Chemical Immunology and the Oncode Initiative (to J.N. and H.O.) and a European Research Council (ERC) Starting Grant to 639005 (S.I.v.K.).
Reviewer information
Nature Reviews Chemistry thanks A. Esser-Kahn and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
S.I.v.K., J.N. and H.O. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Antibody–drug conjugates
-
(ADCs). Constructed of an antibody covalently linked to a small-molecule drug. The conjugation renders the drug inactive, and it is released only at the site targeted by the antibody. This should result in lower background toxicity.
- Antigen
-
A molecule that is capable of inducing an immune response.
- Co-stimulation
-
When an antigen-presenting cell (APC) presents a peptide to a T cell, it can initiate an immune response or an immunosuppressive response. For an immune response, a second set of T cell surface markers (for example, CD80 and CD86) is activated by receptors on the APC to inform the T cell that the peptide presented is dangerous. This second signal is called co-stimulation or signal 2.
- Immune checkpoints
-
Signals — either from T cells or APCs — that initiate an immunosuppressive response. They are often exploited by tumours to stop an antitumour immune response.
- Checkpoint inhibitors
-
Antibodies that block the immunosuppressive response triggered by immune checkpoints. This allows an immune response against a tumour to be re-activated.
- ‘Click-to-release’ chemistry
-
A chemical deprotection strategy based on the inverse-electron-demand Diels–Alder ligation. After the initial ligation of an allylic trans-cyclooctene to a tetrazine, the intermediate pyridazine can rearrange to warrant the elimination (release) of the allylic substituent. Carbamates, as well as ethers and esters, can be deprotected under biocompatible conditions in this manner126.
- Immunophilins
-
Peptidyl–prolyl isomerase enzymes that ensure the proper folding of target proteins by interconverting between the cis and trans bonds of target prolines.
- Ipilimumab
-
A first-generation checkpoint inhibitor. It targets the cytotoxic T lymphocyte antigen 4 (CTLA4) receptor on T cells, which usually binds the co-stimulatory signal on APCs without activating the T cell. The exact immunomodulatory action of this antibody is not yet fully understood.
- Lipocalins
-
Small proteins that transport small hydrophobic molecules.
- Nanobodies
-
Truncated antibodies consisting of a single immunoglobulin domain (12–15 kDa), usually derived from camelids. Their small size makes them easier to express and gives them a shorter half-life (~20 minutes) than full-sized antibodies (>180 kDa; half-life of 5 days or more).
- Bispecific antibodies
-
Constructs that contain two different antigen-targeting domains in a single molecule. They are made by chemical or genetic fusion of different antibodies and can be used to bring two cells into proximity.
- Cytokines
-
Small proteins that regulate the strength and shape of the immune response via intercellular signalling in the immune system. The immune response against a virus, bacteria or helminth is characterized by different cytokines, which can suppress or activate other immune cells and are thus categorized as pro-inflammatory or immunosuppressive.
- Pictet–Spengler reaction
-
The ring-closing reaction between a β-arylethylamine and a ketone or aldehyde to yield a spirocyclic product127.
- Single-chain variable fragments
-
Constructs created by linking genetic material encoding the variable fragment of one antibody heavy chain to the genetic code for the variable fragment of a light chain. They are the smallest fragments of a two-chain antibody that can be constructed.
- T cells
-
Cells that play an essential role in the cellular immune response by recognizing peptides displayed on major histocompatibility proteins (MHCs) of other cells. T cells can be distinguished from other immune cells by the presence of a T cell receptor on their surface. Some T cells kill the cell they recognize, whereas others help the cell presenting the peptide to kill an intracellular pathogen128.
- Therapeutic window
-
The concentration range in which a drug can be used. At the lower end, the drug is not effective, and at the top end, the drug is too toxic to use. A small therapeutic window reduces the usefulness of a drug.
- Transcytosis
-
A form of vesicular transport that carries macromolecules across the interior of the cell.
Rights and permissions
About this article
Cite this article
van Kasteren, S.I., Neefjes, J. & Ovaa, H. Creating molecules that modulate immune responses. Nat Rev Chem 2, 184–193 (2018). https://doi.org/10.1038/s41570-018-0023-9
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41570-018-0023-9
This article is cited by
-
Immunomodulatory Function of Egg White Peptides in RAW264.7 Macrophage Cells and Immunosuppressive Mice Induced by Cyclophosphamide
International Journal of Peptide Research and Therapeutics (2022)