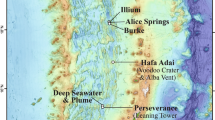
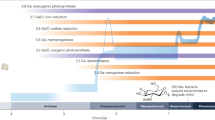
Abstract
Active hydrothermal vents are oases for productivity in the deep ocean, but the flow of dissolved substrates that fuel such abundant life ultimately ceases, leaving behind inactive mineral deposits. The rates of microbial activity on these deposits are largely unconstrained. Here we show primary production occurs on inactive hydrothermal deposits and quantify its contribution to new organic carbon production in the deep ocean. Measured incorporation of 14C-bicarbonate shows that microbial communities on inactive deposits fix inorganic carbon at rates comparable to those on actively venting deposits. Single-cell uptake experiments and nanoscale secondary ion mass spectrometry showed chemoautotrophs comprise a large fraction (>30%) of the active microbial cells. Metagenomic and lipidomic surveys of inactive deposits further revealed that the microbial communities are dominated by Alphaproteobacteria and Gammaproteobacteria using the Calvin–Benson–Bassham pathway for carbon fixation. These findings establish inactive vent deposits as important sites for microbial activity and organic carbon production on the seafloor.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw metagenomic data are available in the NCBI Sequence Read Archive (SRA) under BioProject PRJNA998752.
References
Hannington, M., Jamieson, J., Monecke, T., Petersen, S. & Beaulieu, S. The abundance of seafloor massive sulfide deposits. Geology 39, 1155–1158 (2011).
Haymon, R. M. Growth history of hydrothermal black smoker chimneys. Nature 301, 695–698 (1983).
Van Dover, C. L. in The Ecology of Deep-Sea Hydrothermal Vents (Princeton Univ. Press, 2021).
Jannasch, H. W. & Wirsen, C. O. Chemosynthetic primary production at East Pacific sea floor spreading centers. Bioscience 29, 592–598 (1979).
Corliss, J. B. et al. Submarine thermal springs on the Galapagos Rift. Science 203, 1073–1083 (1979).
Jamieson, J. W. et al. Sulfide geochronology along the Endeavour Segment of the Juan de Fuca Ridge. Geochem. Geophys. Geosyst. 14, 2084–2099 (2013).
Cherkashov, G. et al. Sulfide geochronology along the northern equatorial Mid-Atlantic Ridge. Ore Geol. Rev. 87, 147–154 (2017).
Jamieson, J. W., Galley, C., McNeil, N. & Mora, D. S. Evaluating episodicity of high-temperature venting within seafloor hydrothermal vent fields. Earth Planet. Sci. Lett. 606, 118051 (2023).
Lalou, C., Reyss, J. L. & Brichet, E. Age of sub-bottom sulfide samples at the TAG active mound. In Proc. Ocean Drilling Program 158 (Texas A&M University, 1998).
Kuznetsov, V. et al. The oldest seafloor massive sulfide deposits at the Mid-Atlantic Ridge: Th/U chronology and composition. Geochronometria 42, 100–106 (2015).
Kuznetsov, V. et al. 230Th/U chronology of ore formation within the Semyenov hydrothermal district (13° 31′ N) at the Mid-Atlantic ridge. Geochronometria 38, 72–76 (2011).
Jamieson, J. W. & Gartman, A. Defining active, inactive, and extinct seafloor massive sulfide deposits. Mar. Policy 117, 103926 (2020).
Petersen, S. et al. News from the seabed—geological characteristics and resource potential of deep-sea mineral resources. Mar. Policy 70, 175–187 (2016).
Sylvan, J. B., Toner, B. M. & Edwards, K. J. Life and death of deep-sea vents: bacterial diversity and ecosystem succession on inactive hydrothermal sulfides. MBio 3, e00279–00211 (2012).
Van Dover, C. L. Inactive sulfide ecosystems in the deep sea: a review. Front. Mar. Sci. 6, 461 (2019).
Hou, J. et al. Microbial succession during the transition from active to inactive stages of deep-sea hydrothermal vent sulfide chimneys. Microbiome 8, 1–18 (2020).
Kato, S. et al. Biogeography and biodiversity in sulfide structures of active and inactive vents at deep-sea hydrothermal fields of the Southern Mariana Trough. Appl. Environ. Microbiol. 76, 2968–2979 (2010).
Kato, S. et al. Potential for biogeochemical cycling of sulfur, iron and carbon within massive sulfide deposits below the seafloor. Environ. Microbiol. 17, 1817–1835 (2015).
Sylvan, J. B. et al. Low temperature geomicrobiology follows host rock composition along a geochemical gradient in Lau Basin. Front. Microbiol. 4, 61 (2013).
Toner, B. M. et al. Mineralogy drives bacterial biogeography of hydrothermally inactive seafloor sulfide deposits. Geomicrobiol. J. 30, 313–326 (2013).
Reeves, E. P. et al. Microbial lipids reveal carbon assimilation patterns on hydrothermal sulfide chimneys. Environ. Microbiol. 16, 3515–3532 (2014).
Meier, D. V. et al. Microbial metal‐sulfide oxidation in inactive hydrothermal vent chimneys suggested by metagenomic and metaproteomic analyses. Environ. Microbiol. 21, 682–701 (2019).
Dong, X. et al. Functional diversity of microbial communities in inactive seafloor sulfide deposits. FEMS Microbiol. Ecol. 97, fiab108 (2021).
Orcutt, B. N. et al. Impacts of deep‐sea mining on microbial ecosystem services. Limnol. Oceanogr. 65, 1489–1510 (2020).
Van Dover, C. L. et al. Research is needed to inform environmental management of hydrothermally inactive and extinct polymetallic sulfide (PMS) deposits. Mar. Policy 121, 104183 (2020).
Fornari, D. J. et al. The East Pacific Rise between 9 N and 10 N: twenty-five years of integrated, multidisciplinary oceanic spreading center studies. Oceanography 25, 18–43 (2012).
McAllister, S. M. et al. The Fe (II)-oxidizing Zetaproteobacteria: historical, ecological and genomic perspectives. FEMS Microbiol. Ecol. 95, fiz015 (2019).
Kato, S. et al. Genome‐enabled metabolic reconstruction of dominant chemosynthetic colonizers in deep‐sea massive sulfide deposits. Environ. Microbiol. 20, 862–877 (2018).
Pan, J. et al. Genome-resolved evidence for functionally redundant communities and novel nitrogen fixers in the deyin-1 hydrothermal field, Mid-Atlantic Ridge. Microbiome 10, 1–20 (2022).
Günter, J., Zubkov, M. V., Yakushev, E., Labrenz, M. & Jürgens, K. High abundance and dark CO2 fixation of chemolithoautotrophic prokaryotes in anoxic waters of the Baltic Sea. Limnol. Oceanogr. 53, 14–22 (2008).
Herndl, G. J. et al. Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl. Environ. Microbiol. 71, 2303–2309 (2005).
Dyksma, S. et al. Ubiquitous Gammaproteobacteria dominate dark carbon fixation in coastal sediments. ISME J. 10, 1939–1953 (2016).
McNichol, J. et al. Genus-specific carbon fixation activity measurements reveal distinct responses to oxygen among hydrothermal vent Campylobacteria. Appl. Environ. Microbiol. 88, e02083–02021 (2022).
Olins, H., Rogers, D., Frank, K., Vidoudez, C. & Girguis, P. Assessing the influence of physical, geochemical and biological factors on anaerobic microbial primary productivity within hydrothermal vent chimneys. Geobiology 11, 279–293 (2013).
Bonch-Osmolovskaya, E. A. et al. Activity and distribution of thermophilic prokaryotes in hydrothermal fluid, sulfidic structures, and sheaths of alvinellids (East Pacific Rise, 13 N). Appl. Environ. Microbiol. 77, 2803–2806 (2011).
Wirsen, C. O., Jannasch, H. W. & Molyneaux, S. J. Chemosynthetic microbial activity at Mid‐Atlantic Ridge hydrothermal vent sites. J. Geophys. Res. Solid Earth 98, 9693–9703 (1993).
Tuttle, J., Wirsen, C. & Jannasch, H. Microbial activities in the emitted hydrothermal waters of the Galapagos Rift vents. Mar. Biol. 73, 293–299 (1983).
Mandernack, K. W. & Tebo, B. M. In situ sulfide removal and CO2 fixation rates at deep-sea hydrothermal vents and the oxic/anoxic interface in Framvaren Fjord, Norway. Mar. Chem. 66, 201–213 (1999).
Wirsen, C. O., Tuttle, J. & Jannasch, H. Activities of sulfur-oxidizing bacteria at the 21 N East Pacific Rise vent site. Mar. Biol. 92, 449–456 (1986).
McNichol, J. et al. Primary productivity below the seafloor at deep-sea hot springs. Proc. Natl Acad. Sci. USA 115, 6756–6761 (2018).
Dekas, A. E. et al. Characterizing chemoautotrophy and heterotrophy in marine archaea and bacteria with single-cell multi-isotope NanoSIP. Front. Microbiol. 10, 2682 (2019).
Braun, A. et al. Reviews and syntheses: heterotrophic fixation of inorganic carbon-significant but invisible flux in environmental carbon cycling. Biogeosciences 18, 3689–3700 (2021).
Wang, F. et al. GeoChip-based analysis of metabolic diversity of microbial communities at the Juan de Fuca Ridge hydrothermal vent. Proc. Natl Acad. Sci. USA 106, 4840–4845 (2009).
Hügler, M. & Sievert, S. M. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Annu. Rev. Mar. Sci. 3, 261–289 (2011).
Sievert, S. M. & Vetriani, C. Chemoautotrophy at deep-sea vents: past, present, and future. Oceanography 25, 218–233 (2012).
House, C. H., Schopf, J. W. & Stetter, K. O. Carbon isotopic fractionation by Archaeans and other thermophilic prokaryotes. Org. Geochem. 34, 345–356 (2003).
Havig, J. R., Raymond, J., Meyer-Dombard, D. A. R., Zolotova, N. & Shock, E. L. Merging isotopes and community genomics in a siliceous sinter-depositing hot spring. J. Geophys. Res. Biogeosciences 116, G01005 (2011).
Khachikyan, A. et al. Direct cell mass measurements expand the role of small microorganisms in nature. Appl. Environ. Microbiol. 85, e00493–00419 (2019).
Hu, S. K. et al. Protistan grazing impacts microbial communities and carbon cycling at deep-sea hydrothermal vents. Proc. Natl Acad. Sci. USA 118, e2102674118 (2021).
Boschen, R. E., Rowden, A. A., Clark, M. R. & Gardner, J. P. Mining of deep-sea seafloor massive sulfides: a review of the deposits, their benthic communities, impacts from mining, regulatory frameworks and management strategies. Ocean Coast. Manag. 84, 54–67 (2013).
Neufeld, M., Metaxas, A. & Jamieson, J. W. Non-vent megafaunal communities on the Endeavour and Middle Valley segments of the Juan de Fuca Ridge, Northeast Pacific Ocean. Front. Mar. Sci. 9, 804 (2022).
Beaulieu, S. & Szafranski, K. InterRidge Global Database of Active Submarine Hydrothermal Vent Fields Version 3.4. World Wide Web Electronic Publication (accessed 2020); http://vents-data.interridge.org
Minami, H. & Ohara, Y. The Gondou hydrothermal field in the Ryukyu Arc: a huge hydrothermal system on the flank of a caldera volcano. Geochem. Geophys. Geosyst. 18, 3489–3516 (2017).
Jamieson, J., Clague, D. & Hannington, M. D. Hydrothermal sulfide accumulation along the Endeavour segment, Juan de Fuca Ridge. Earth Planet. Sci. Lett. 395, 136–148 (2014).
Fornari, D. J., Haymon, R. M., Perfit, M. R., Gregg, T. K. & Edwards, M. H. Axial summit trough of the East Pacific Rice 9°–10° N: geological characteristics and evolution of the axial zone on fast spreading mid‐ocean ridge. J. Geophys. Res. Solid Earth 103, 9827–9855 (1998).
Wu, J. N. et al. Extent and volume of lava flows erupted at 9 50′ N, East Pacific Rise in 2005–2006 from autonomous underwater vehicle surveys. Geochem. Geophys. Geosyst. 23, e2021GC010213 (2022).
Monteverde, D. R. et al. Distribution of extracellular flavins in a coastal marine basin and their relationship to redox gradients and microbial community members. Environ. Sci. Technol. 52, 12265–12274 (2018).
Molari, M., Manini, E. & Dell’Anno, A. Dark inorganic carbon fixation sustains the functioning of benthic deep‐sea ecosystems. Glob. Biogeochem. Cycles 27, 212–221 (2013).
McNichol, J. et al. Assessing microbial processes in deep-sea hydrothermal systems by incubation at in situ temperature and pressure. Deep Sea Res. I 115, 221–232 (2016).
Meyer, N. R., Fortney, J. L. & Dekas, A. E. NanoSIMS sample preparation decreases isotope enrichment: magnitude, variability and implications for single‐cell rates of microbial activity. Environ. Microbiol. 23, 81–98 (2021).
Polerecky, L. et al. Look@ NanoSIMS–a tool for the analysis of nanoSIMS data in environmental microbiology. Environ. Microbiol. 14, 1009–1023 (2012).
Parada, A. E. et al. Constraining the composition and quantity of organic matter used by abundant marine Thaumarchaeota. Environ. Microbiol. 25, 689–704 (2023).
Holmes, R. M., Aminot, A., Kérouel, R., Hooker, B. A. & Peterson, B. J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 56, 1801–1808 (1999).
Rosman, K. & Taylor, P. Isotopic compositions of the elements 1997 (Technical Report). Pure Appl. Chem. 70, 217–235 (1998).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, D., Liu, C.-M., Luo, R., Sadakane, K. & Lam, T.-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 11, 119 (2010).
Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Kanehisa, M., Sato, Y. & Morishima, K. BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J. Mol. Biol. 428, 726–731 (2016).
Garber, A. I. et al. FeGenie: a comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Front. Microbiol. 11, 13 (2020).
Garber, A., Ramirez, G. A., Merino, N., Pavia M. J., McAllister, S. M. MagicLamp: toolkit for annotation of genomic data using discreet and curated HMM sets. MagicLamp, GitHub repository (2023); https://github.com/Arkadiy-Garber/MagicLamp
Sturt, H. F., Summons, R. E., Smith, K., Elvert, M. & Hinrichs, K. U. Intact polar membrane lipids in prokaryotes and sediments deciphered by high‐performance liquid chromatography/electrospray ionization multistage mass spectrometry—new biomarkers for biogeochemistry and microbial ecology. Rapid Commun. Mass Spectrom. 18, 617–628 (2004).
Meador, T. B., Zhu, C., Elling, F. J., Könneke, M. & Hinrichs, K.-U. Identification of isoprenoid glycosidic glycerol dibiphytanol diethers and indications for their biosynthetic origin. Org. Geochem. 69, 70–75 (2014).
Wörmer, L., Lipp, J. S., Schröder, J. M. & Hinrichs, K.-U. Application of two new LC–ESI–MS methods for improved detection of intact polar lipids (IPLs) in environmental samples. Org. Geochem. 59, 10–21 (2013).
Acknowledgements
We thank the captain and shipboard crew of the RV Atlantis during the AT42-09 and AT42-21 expeditions and of the RV Roger Revelle during the RR2102 expedition. We are also thankful for the input and hard work of the pilots and crew of the HOV Alvin, ROV Jason and AUV Sentry; the science done for this project would not be possible without the support of and collaboration with University–National Oceanographic Laboratory System operators and vessels and the National Deep Submergence Facility at Woods Hole Oceanographic Institution. This work was supported by grants from the US National Science Foundation to J.B.S. (OCE 1756339), B.M.T. (OCE 1756558), M.K.T. (OCE 1756419), D.J.F. (OCE 1949485) and A.E.D. (NSF CAREER award 2143035). J.B.S. acknowledges support from a Simons Foundation Early Career Investigator in Aquatic Microbial Ecology and Evolution Award. J.J. acknowledges financial support from the Canada Research Chairs program (CRC-2020-001165). S.M. acknowledges funding support from the Natural Sciences and Engineering Research Council of Canada Collaborative Research and Training Experience project in Marine Geodynamics and Georesources program. E.P.R. acknowledges funding support from the University of Bergen Meltzer Fund. We thank Emily Paris in the Dekas Laboratory for performing the ammonium quantification. The Stanford Nano Shared Facilities where the nanoSIMS analyses were conducted is supported in part by the National Science Foundation under award ECCS-2026822. F.S., J.B. and A.M. acknowledge funding from the German Research Foundation (DFG) under Germany’s Excellence Strategy—EXC 2077-390741603. Finally, thank you to T. Meek (Texas A&M University) for providing access to liquid scintillation counting equipment.
Author information
Authors and Affiliations
Contributions
J.B.S., B.M.T., M.K.T. and A.M.A. conceived the study. A.M.A., J.B.S., J.J., A.E.D. and F.S. wrote the manuscript with input from all authors. D.J.F. contributed site survey information and provided support for field work. A.M.A., R.J., C.P.H., E.P.R. and J.B. performed sample collection. A.M.A. performed carbon fixation measurements and conducted bioinformatic analysis. R.J. contributed scanning electron microscopy images. J.J. and S.M. performed mineralogical analysis. A.E.D. and N.R.M. performed NanoSIMS analysis. F.S. and A.M. performed analysis of TOC and PLFA. C.P.H. performed cell counts. All authors reviewed and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
a, Representative samples collected from inactive and active hydrothermal vent deposits including photographs of bulk samples (A1-C1) as well as thin section micrographs viewed with transmitted (A2-C2) and reflected light (A3-C3); Anh, anhydrite; Atc, atacamite; Cpy,chalcopyrite; Fe-oxy, iron oxyhydroxide; Mc, marcasite, Py, pyrite; Ps, pore space. b, Bulk minerology of samples. Black denotes iron oxyhydroxides, Yellow denotes sulfides, and Blue denotes sulfates. c, Photograph of representative subdivided inactive vent sample from Lucky’s Mound used in carbon assimilation assays showing the three regions of interest (inner conduit, middle region, and outer crust).
Extended Data Fig. 3
NanoSIMS comparison of uptake of 13C−bicarbonate a, and 15N−ammonium b, in individual cells (gray dots) from a single rock sample at each of AlvinellidRock (active vent deposit) and Mvent (inactive vent deposit) after 2 days of incubation. Box plots indicating the median, upper, and lower quartiles (but, appearing as lines due to mostly low activity/inactive cells) are overlaid in black. Numbers above the plots indicate the number of regions of interest (putative cells) analyzed, and asterisks indicate a statistically significant difference (p < 0.05) using a two-sided, Mann-Whitney (MW) test with a Bonferroni correction for multiple hypotheses.
Extended Data Fig. 4
Relative uptake of 13C-bicarbonate and 15N−ammonium in individual cells (black circles) measured by nanoSIMS from AlvinellidRock (active vent deposit) and Mvent (inactive vent deposit) after 2 days of incubation. Data is replotted from main text Fig. 3 with truncated axes to better display data points around the origin (lower activity cells). Based on their relative assimilation of each substrate, the regions below the 2:1 line include chemoautotrophic cells, the region above the 2:1 line includes heterotrophic cells, and the regions to the left (x-axis) or below (y-axis) the dashed lines include cells below the detection limit of uptake for that substrate. Regions are defined as in Dekas et al.41.
Extended Data Fig. 6
Summary of published carbon fixation rates from active hydrothermal vent mineral deposits (black; right-axis) and hydrothermal vent fluids (grey; left-axis). The blue and orange dots denote the highest and lowest reported rates, respectively.
Supplementary information
Supplementary Tables 1–3
LithoGenie HMM list as implemented within MagicLamp (taken from https://github.com/Arkadiy-Garber/MagicLamp). List of metagenome-assembled genomes from inactive hydrothermal vent deposits showing the presence (indicated with a +) of key genes for the carbon fixation pathways examined (Extended Data Table 2). Cell-normalized carbon fixation rate mean and ranges from this work and other deep-sea environments.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Achberger, A.M., Jones, R., Jamieson, J. et al. Inactive hydrothermal vent microbial communities are important contributors to deep ocean primary productivity. Nat Microbiol 9, 657–668 (2024). https://doi.org/10.1038/s41564-024-01599-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41564-024-01599-9
This article is cited by
-
Inactive vents, active producers
Nature Reviews Microbiology (2024)
-
Mineral-eating microorganisms at extinct hydrothermal vents
Nature Microbiology (2024)