Abstract
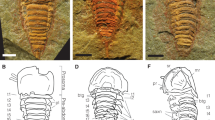
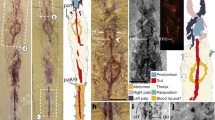
Understanding the genealogical relationships among the arachnid orders is an onerous task, but fossils have aided in anchoring some branches of the arachnid tree of life. The discovery of Palaeozoic fossils with characters found in both extant spiders and other arachnids provided evidence for a series of extinctions of what was thought to be a grade, Uraraneida, that led to modern spiders. Here, we report two extraordinarily well-preserved Mesozoic members of Uraraneida with a segmented abdomen, multi-articulate spinnerets with well-defined spigots, modified male palps, spider-like chelicerae and a uropygid-like telson. The new fossils, belonging to the species Chimerarachne yingi, were analysed phylogenetically in a large data matrix of extant and extinct arachnids under a diverse regime of analytical conditions, most of which resulted in placing Uraraneida as the sister clade of Araneae (spiders). The phylogenetic placement of this arachnid fossil extends the presence of spinnerets and modified palps more basally in the arachnid tree than was previously thought. Ecologically, the new fossil extends the record of Uraraneida 170 million years towards the present, thus showing that uraraneids and spiders co-existed for a large fraction of their evolutionary history.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Wheeler, W. C. et al. The spider tree of life: phylogeny of Araneae based on target-gene analyses from an extensive taxon sampling. Cladistics 33, 574–616 (2017).
Dunlop, J. A. Geological history and phylogeny of Chelicerata. Arthropod Struct. Dev. 39, 124–142 (2010).
Garrison, N. L. et al. Spider phylogenomics: untangling the spider tree of life. PeerJ 4, e1719 (2016).
Shear, W. A., Palmer, J. M., Coddington, J. A. & Bonamo, P. M. A Devonian spinneret: early evidence of spiders and silk use. Science 246, 479–481 (1989).
Selden, P. A., Shear, W. A. & Bonamo, P. M. A spider and other arachnids from the Devonian of New York, and reinterpretations of Devonian Araneae. Palaeontology 34, 241–281 (1991).
Selden, P. A., Shear, W. A. & Sutton, M. D. Fossil evidence for the origin of spider spinnerets, and a proposed arachnid order. Proc. Natl. Acad. Sci. USA 105, 20781–20785 (2008).
Garwood, R. J. et al. Almost a spider: a 305-million-year-old fossil arachnid and spider origins. Proc. R. Soc. B Biol. Sci. 283, 20160125 (2016).
Eskov, K. Y. & Selden, P. A. First record of spiders from the Permian period (Araneae: Mesothelae). Bull. Br. Arachnol. Soc. 13, 111–116 (2005).
Selden, P. A. A redescription of Juraraneus rasnitsyni Eskov, 1984 (Araneae: Juraraneidae), from the Jurassic of Russia. Bull. Br. Arachnol. Soc. 15, 315–321 (2012).
Shear, W. A., Selden, P. A., Rolfe, W. D. I., Bonamo, P. M. & Grierson, J. D. New terrestrial arachnids from the Devonian of Gilboa, New York (Arachnida, Trigonotarbida). Am. Mus. Novit. 2901, 1–74 (1987).
Giribet, G., Edgecombe, G. D., Wheeler, W. C. & Babbitt, C. Phylogeny and systematic position of Opiliones: a combined analysis of chelicerate relationships using morphological and molecular data. Cladistics 18, 5–70 (2002).
Garwood, R. J., Dunlop, J. A., Giribet, G. & Sutton, M. D. Anatomically modern Carboniferous harvestmen demonstrate early cladogenesis and stasis in Opiliones. Nat. Commun. 2, 444 (2011).
Garwood, R. J., Dunlop, J. A., Knecht, B. J. & Hegna, T. A. The phylogeny of fossil whip spiders. BMC Evol. Biol. 17, 105 (2017).
Wang, B. et al. Cretaceous arachnid Chimerarachne yingi gen. et sp. nov. illuminates spider origins. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-017-0449-3 (2018).
Ross, A., Mellish, C., York, P. & Crighton, B. in Biodiversity of Fossil in Amber from the Major World Deposits (ed. Penney, D.) 208–235 (Siri Scientific Press, Rochdale, 2010).
Shi, G. et al. Age constraint on Burmese amber based on U–Pb dating of zircons. Cretac. Res. 37, 155–163 (2012).
Prendini, L. Species or supraspecific taxa as terminals in cladistic analysis? Groundplans versus exemplars revisited. Syst. Biol. 50, 290–300 (2001).
Shultz, J. W. A phylogenetic analysis of the arachnid orders based on morphological characters. Zool. J. Linn. Soc. 150, 221–265 (2007).
Garwood, R. J. & Dunlop, J. Three-dimensional reconstruction and the phylogeny of extinct chelicerate orders. PeerJ 2, e641 (2014).
Xu, X. et al. A genus-level taxonomic review of primitively segmented spiders (Mesothelae, Liphistiidae). ZooKeys 488, 121–151 (2015).
Giribet, G. & Dunlop, J. A. First identifiable Mesozoic harvestman (Opiliones: Dyspnoi) from Cretaceous Burmese amber. Proc. R. Soc. B Biol. Sci. 272, 1007–1013 2005).
Poinar, G. in Advances in Arachnology and Developmental Biology. Papers Dedicated to Prof. Dr. Bozidar Curcic Vol. Monographs, 12 (eds Makarov, S. E. & Dimitrijevic, R. N.) 267–274 (Inst. Zool., Belgrade; BAS, Sofia; Fac. Life Sci., Vienna; SASA, Belgrade and UNESCO MAB Serbia, 2008).
Engel, M. S. et al. The first Mesozoic microwhip scorpion (Palpigradi): a new genus and species in mid-Cretaceous amber from Myanmar. Sci. Nat. 103, 19 (2016).
Dunlop, J. A., Bird, T. L., Brookhart, J. O. & Bechly, G. A camel spider from Cretaceous Burmese amber. Cretac. Res. 56, 265–273 (2015).
Henderickx, H. & Boone, M. The basal pseudoscorpion family Feaellidae Ellingsen, 1906 walks the earth for 98.000.000 years: a new fossil genus has been found in Cretaceous Burmese amber (Pseudoscorpiones: Feaellidae). Publicatie 27, 7–12 (2016).
Selden, P. A., Dunlop, J. A., Giribet, G., Zhang, W. & Ren, D. The oldest armoured harvestman (Arachnida: Opiliones: Laniatores), from Upper Cretaceous Myanmar amber. Cretaceous Res. 65, 206–212 (2016).
Cai, C. & Huang, D. A new genus of whip-scorpions in Upper Cretaceous Burmese amber: earliest fossil record of the extant subfamily Thelyphoninae (Arachnida: Thelyphonida: Thelyphonidae). Cretaceous Res. 69, 100–105 (2017).
Wunderlich, J. Description of the first fossil Ricinulei in amber from Burma (Myanmar), the first report of this arachnid order from the Mesozoic and from Asia, with notes on the related extinct order Trigonotarbida. Beitr. Zur. Araneol. 7, 233–244 (2012).
Selden, P. A. & Ren, D. A review of Burmese amber arachnids. J. Arachnol. 45, 324–343 (2017).
Murienne, J., Benavides, L. R., Prendini, L., Hormiga, G. & Giribet, G. Forest refugia in Western and Central Africa as ‘museums’ of Mesozoic biodiversity. Biol. Lett. 9, 20120932 (2013).
Selden, P. A. First fossil mesothele spider, from the Carboniferous of France. Rev. Suisse Zool. 2, 585–596 (1996).
Wolfe, J. M., Daley, A. C., Legg, D. A. & Edgecombe, G. D. Fossil calibrations for the arthropod tree of life. Earth-Sci. Rev. 160, 43–110 (2016).
Goloboff, P. A. & Catalano, S. A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32, 221–238 (2016).
Goloboff, P. A. Estimating character weights during tree search. Cladistics 9, 83–91 (1993).
Goloboff, P. A. Extended implied weighting. Cladistics 30, 260–272 (2014).
Ronquist, F. et al. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542 (2012).
Lewis, P. O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001).
Acknowledgements
Financial support was provided by the Strategic Priority Research Program (B) (XDB18000000) and the programme Macroevolutionary Processes and Palaeoenvironments of Major Historical Biota (XDPB05) of the Chinese Academy of Sciences, the National Natural Science Foundation of China (41688103 and 91514302) and US National Science Foundation grants 1457300 (to G.H.) and 1457539 (to G.G.) (Collaborative Research: Phylogeny and diversification of the orb weaving spiders (Araneae)). We are grateful to J. Sun for reconstruction and S. Wu for micro computed tomography assistance. Published by a grant from the Wetmore Colles fund.
Author information
Authors and Affiliations
Contributions
D.H., G.G., G.H., C.C., Y.S., Z.Y. and F.X. participated in the morphological studies. D.H. coordinated the study. G.G. and G.H. conducted the phylogenetic analyses. G.G., D.H. and G.H. prepared the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary figures, description, characters and references.
Rights and permissions
About this article
Cite this article
Huang, D., Hormiga, G., Cai, C. et al. Origin of spiders and their spinning organs illuminated by mid-Cretaceous amber fossils. Nat Ecol Evol 2, 623–627 (2018). https://doi.org/10.1038/s41559-018-0475-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41559-018-0475-9
This article is cited by
-
The complete mitochondrial genome of the intertidal spider (Desis jiaxiangi) provides novel insights into the adaptive evolution of the mitogenome and the evolution of spiders
BMC Ecology and Evolution (2021)
-
Morphological changes during the post-embryonic ontogeny of mesothelan spiders and aspects of character evolution in early spiders
Development Genes and Evolution (2021)
-
Developmental gene expression as a phylogenetic data class: support for the monophyly of Arachnopulmonata
Development Genes and Evolution (2020)
-
Increasing species sampling in chelicerate genomic-scale datasets provides support for monophyly of Acari and Arachnida
Nature Communications (2019)