Abstract
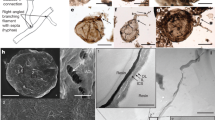
Fungi have recently been found to comprise a significant part of the deep biosphere in oceanic sediments and crustal rocks. Fossils occupying fractures and pores in Phanerozoic volcanics indicate that this habitat is at least 400 million years old, but its origin may be considerably older. A 2.4-billion-year-old basalt from the Palaeoproterozoic Ongeluk Formation in South Africa contains filamentous fossils in vesicles and fractures. The filaments form mycelium-like structures growing from a basal film attached to the internal rock surfaces. Filaments branch and anastomose, touch and entangle each other. They are indistinguishable from mycelial fossils found in similar deep-biosphere habitats in the Phanerozoic, where they are attributed to fungi on the basis of chemical and morphological similarities to living fungi. The Ongeluk fossils, however, are two to three times older than current age estimates of the fungal clade. Unless they represent an unknown branch of fungus-like organisms, the fossils imply that the fungal clade is considerably older than previously thought, and that fungal origin and early evolution may lie in the oceanic deep biosphere rather than on land. The Ongeluk discovery suggests that life has inhabited submarine volcanics for more than 2.4 billion years.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Edwards, K. J., Becker, K. & Colwell, F. The deep, dark energy biosphere: intraterrestrial life on Earth. Annu. Rev. Earth Planet. Sci. 40, 551–568 (2012).
Schumann, G., Manz, W., Reitner, J. & Lustrino, M. Ancient fungal life in North Pacific Eocene oceanic crust. Geomicrobiol. J. 21, 241–246 (2004).
Peckmann, J., Bach, W., Behrens, K. & Reitner, J. Putative cryptoendolithic life in Devonian pillow basalt, Rheinisches Schiefergebirge, Germany. Geobiology 6, 125–135 (2008).
Eickmann, B., Bach, W., Kiel, S., Reitner, J. & Peckmann, J. Evidence for cryptoendolithic life in Devonian pillow basalts of Variscan orogens, Germany. Palaeogeogr. Palaeoclimatol. Palaeoecol. 283, 120–125 (2009).
Ivarsson, M. et al. Fossilized fungi in subseafloor Eocene basalts. Geology 40, 163–166 (2012).
Ivarsson, M., Bengtson, S., Skogby, H., Belivanova, V. & Marone, F. Fungal colonies in open fractures of subseafloor basalt. Geo-Mar. Lett. 33, 233–243 (2013).
Ivarsson, M. et al. Zygomycetes in vesicular basanites from Vesteris Seamount, Greenland Basin – a new type of cryptoendolithic fungi. PLoS ONE 10, e0133368 (2015).
Furnes, H . et al. in Modern Approaches in Solid Earth Sciences Vol. 4 (eds Dilek, Y., Furnes, H. & Muehlenbachs, K .) 1–68 (Springer, 2008).
Grosch, E. G. & McLoughlin, N. Reassessing the biogenicity of Earth’s oldest trace fossil with implications for biosignatures in the search for early life. Proc. Natl Acad. Sci. USA 111, 8380–8385 (2014).
Staudigel, H., Furnes, H. & DeWit, M. Paleoarchean trace fossils in altered volcanic glass. Proc. Natl Acad. Sci. USA 112, 6892–6897 (2015).
Grosch, E. G. & McLoughlin, N. Questioning the biogenicity of titanite mineral trace fossils in Archean pillow lavas. Proc. Natl Acad. Sci. USA 112, E1390–E1391 (2015).
McLoughlin, N. & Grosch, E. G. A hierarchical system for evaluating the biogenicity of metavolcanic- and ultramafic-hosted microalteration textures in the search for extraterrestrial life. Astrobiology 15, 901–921 (2015).
Fisk, M. & McLoughlin, N. Atlas of alteration textures in volcanic glass from the ocean basins. Geosphere 39, 317–341 (2013).
Phillips, W. J. Interpretation of crystalline spheroidal structures in igneous rocks. Lithos 6, 235–244 (1973).
Mcloughlin, N., Staudigel, H., Furnes, H., Eickmann, B. & Ivarsson, M. Mechanisms of microtunneling in rock substrates: distinguishing endolithic biosignatures from abiotic microtunnels. Geobiology 8, 245–255 (2010).
Lepot, K., Benzerara, K. & Philippot, P. Biogenic versus metamorphic origins of diverse microtubes in 2.7 Gyr old volcanic ashes: multi-scale investigations. Earth Planet. Sci. Lett. 312, 37–47 (2011).
Pasteris, J. D. & Wopenka, B. Necessary, but not sufficient: Raman identification of disordered carbon as a signature of ancient life. Astrobiology 3, 727–738 (2003).
Sumner, D. Y. Poor preservation potential of organics in Meridiani Planum hematite-bearing sedimentary rocks. J. Geophys. Res. 109, E12007 (2004).
Chi Fru, E. et al. Fossilized iron bacteria reveal pathway to biological origin of banded iron formation. Nat. Commun. 4, 2050 (2013).
Staudigel, H., Hart, S. R. & Richardson, S. H. Alteration of the oceanic crust: processes and timing. Earth Planet. Sci. Lett. 52, 311–327 (1981).
Erikson, D. The morphology, cytology, and taxonomy of the Actinomycetes. Annu. Rev. Microbiol. 3, 23–54 (1949).
Higgins, M. L. & Silvey, J. K. G. Slide culture observations of two freshwater Actinomycetes. Trans. Am. Microsc. Soc. 85, 390–398 (1966).
Goodfellow, M . et al. (eds) Bergey's Manual of Systematic Bacteriology Vol. 5: The Actinobacteria 2nd edn (Springer, 2012).
Bull, A. T. in Extremophiles Handbook (eds Horikoshi, K. et al.) 1203–1240 (Springer, 2011).
Edgcomb, V. P., Beaudoin, D., Gast, R., Biddle, J. F. & Teske, A. Marine subsurface eukaryotes: the fungal majority. Environ. Microbiol. 13, 172–183 (2011).
Orsi, W., Biddle, J. F. & Edgcomb, V. Deep sequencing of subseafloor eukaryotic rRNA reveals active fungi across marine subsurface provinces. PLoS ONE 8, e56335 (2013).
Sohlberg, E . et al. Revealing the unexplored fungal communities in deep groundwater of crystalline bedrock fracture zones in Olkiluoto, Finland. Front. Microbiol. 6, 573 (2015).
Ivarsson, M., Bengtson, S. & Neubeck, A. The igneous oceanic crust – Earth’s largest fungal habitat? Fungal Ecol. 20, 249–255 (2016).
Pachiadaki, M. G., Rédou, V., Beaudoin, D. J., Burgaud, G. & Edgcomb, V. P. Fungal and prokaryotic activities in the marine subsurface biosphere at Peru Margin and Canterbury Basin inferred from RNA-based analyses and microscopy. Front. Microbiol. 7, 846 (2016).
Nicolson, T. H. Mycorrhiza in the Gramineae: I. Vesicular-arbuscular endophytes, with special reference to the external phase. J. Brit. Mycolog. Soc. 42, 421–438 (1959).
Glass, N. L., Rasmussen, C., Roca, M. G. & Read, N. D. Hyphal homing, fusion and mycelial interconnectedness. Trends Microbiol. 12, 135–141 (2004).
Bengtson, S. et al. Deep-biosphere consortium of fungi and prokaryotes in Eocene sub-seafloor basalts. Geobiology 12, 489–496 (2014).
Ivarsson, M. et al. A fungal-prokaryotic consortium at the basalt-zeolite interface in subseafloor igneous crust. PLoS ONE 10, e0140106 (2015).
Brown, J. W. & Sorhannus, U. A molecular genetic timescale for the diversification of autotrophic stramenopiles (Ochrophyta): substantive underestimation of putative fossil ages. PLoS ONE 5, e12759 (2010).
Stephenson, L. W., Erwin, D. C. & Leary, J. V. Hyphal anastomosis in Phytophthora capsici. Phytopathology 64, 149–150 (1974).
Sbrana, C., Nuti, M. P. & Giovannetti, M. Self-anastomosing ability and vegetative incompatibility of Tuber borchii isolates. Mycorrhiza 17, 667–675 (2007).
Dowson, C. G., Boddy, L. & Rayner, A. D. M. Development and extension of mycelial cords in soil at different temperatures and moisture contents. Mycol. Res. 92, 383–391 (1989).
Cavalier-Smith, T. & Chao, E. E. The opalozoan Apusomonas is related to the common ancestor of animals, fungi, and choanoflagellates. Proc. R. Soc. Lond. B 261, 1–6 (1995).
James, T. Y. et al. Reconstructing the early evolution of fungi using a six-gene phylogeny. Nature 443, 818–822 (2006).
Baldauf, S. L. An overview of the phylogeny and diversity of eukaryotes. J. Syst. Evol. 46, 263–273 (2008).
Heckman, D. S. et al. Molecular evidence for the early colonization of land by fungi and plants. Science 293, 1129–1133 (2001).
Hedges, S. B., Blair, J. E., Venturi, M. & Shoe, J. L. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol. Biol. 4, 2 (2004).
Padovan, A. C. B., Sanson, G. F. O., Brunstein, A. & Briones, M. R. S. Fungi evolution revisited: application of the penalized likelihood method to a Bayesian fungal phylogeny provides a new perspective on phylogenetic relationships and divergence dates of Ascomycota groups. J. Mol. Evol. 60, 726–735 (2005).
Bhattacharya, D ., Yoon, H. S ., Hedges, S. B. & Hackett, J. D. in The Timetree of Life (eds Hedges, S. B. & Kumar, S. ) 116–120 (Oxford Univ. Press, 2009).
Parfrey, L. W., Lahr, D. J. G., Knoll, A. H. & Katz, L. A. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc. Natl Acad. Sci. USA 108, 13624–13629 (2011).
Taylor, T. N ., Krings, M. & Taylor, E. L. Fossil Fungi (Elsevier, 2015).
Sharpe, S. C ., Eme, L ., Brown, M. W. & Roger, A. J. in Evolutionary Transitions to Multicellular Life (eds Ruiz-Trillo, I. & Nedelcu, A. M. ) 3–29 (Springer, 2015).
Kohlmeyer, J. & Kohlmeyer, E. Marine Mycology (Academic, 1979).
Benton, M. J. et al. Constraints on the timescale of animal evolutionary history. Palaeontol. Electron. 18, 18.1.1FC (2015).
Lanari, P. et al. XMapTools: a MATLAB©-based program for electron microprobe X-ray image processing and geothermobarometry. Comput. Geosci. 62, 227–240 (2014).
Bourdelle, F., Parra, T., Chopin, C. & Beyssac, O. A new chlorite geothermometer for diagenetic to low-grade metamorphic conditions. Contrib. Mineral. Petrol. 165, 723–735 (2013).
Marone, F. & Stampanoni, M. Regridding reconstruction algorithm for real-time tomographic imaging. J. Synchrotron Radiat. 19, 1029–1037 (2012).
Johnson, J. E., Gerpheide, A., Lamb, M. P. & Fischer, W. W. O2 constraints from Paleoproterozoic detrital pyrite and uraninite. GSA Bull. 126, 813–830 (2014).
Acknowledgements
Our work has been supported by the Agouron Institute, Swedish Research Council (2012-4364, 2013-4290), Danish National Research Foundation (DNRF53), Australian Research Council (DP110103660, DP140100512), Paul Scherrer Institute (20130185, 20141047), Australian Microscopy & Microanalysis Research Facility, National Science Foundation (EAR-05-45484), NASA Astrobiology Institute (NNA04CC09A), Natural Sciences and Engineering Research Council, and the European Commission CALIPSO programme (312284). We thank V. Belivanova for technical assistance, P. von Knorring for drafting Supplementary Fig. 6, J. Peckmann for supplying images for Supplementary Fig. 8c,d and A. Tehler for discussions.
Author information
Authors and Affiliations
Contributions
A.B. provided the material and geological information; B.R. discovered the filamentous structures; S.B., B.R. and M.I. designed the study; S.B., B.R., M.I., J.M. and C.B. performed the investigation; S.B. and B.R. wrote the paper with input from other co-authors; and M.S. and F.M. designed and operated the TOMCAT beamline.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Discussion; Supplementary Figures; Supplementary Tables (PDF 32681 kb)
Supplementary Video 1
Ongeluk vesicle with filamentous fossils. SRXTM surface/volume rendering, 32.5 µm thick virtual slice passing through specimen. Note NW-SE-trending thin veins connecting the vesicle with the surroundings. Swedish Museum of Natural History X6137, same vesicle as in Fig. 3. (MPG 25880 kb)
Rights and permissions
About this article
Cite this article
Bengtson, S., Rasmussen, B., Ivarsson, M. et al. Fungus-like mycelial fossils in 2.4-billion-year-old vesicular basalt. Nat Ecol Evol 1, 0141 (2017). https://doi.org/10.1038/s41559-017-0141
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41559-017-0141
This article is cited by
-
Genome characterization of two novel deep-sea sediment fungi, Penicillium pacificagyrus sp. nov. and Penicillium pacificasedimenti sp. nov., from South Pacific Gyre subseafloor sediments, highlights survivability
BMC Genomics (2023)
-
Organic walled microfossils in wet peperites from the early Cretaceous Paraná-Etendeka volcanism of Brazil
Scientific Reports (2023)
-
Eukaryogenesis and oxygen in Earth history
Nature Ecology & Evolution (2022)
-
Biosignatures of ancient microbial life are present across the igneous crust of the Fennoscandian shield
Communications Earth & Environment (2021)
-
Cryptic terrestrial fungus-like fossils of the early Ediacaran Period
Nature Communications (2021)