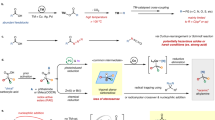
Abstract
Transition-metal–carbonyl complexes are common organometallic reagents that feature metal–CO bonds. These complexes have proven to be powerful catalysts for various applications. By contrast, silicon–carbonyl complexes, organosilicon reagents poised to be eco-friendly alternatives for transition-metal carbonyls, have remained largely elusive. They have mostly been explored theoretically and/or through low-temperature matrix isolation studies, but their instability had typically precluded isolation under ambient conditions. Here we present the synthesis, isolation and full characterization of stable silyl-substituted silicon–carbonyl complexes, along with bonding analysis. Initial reactivity investigations showed examples of CO liberation, which could be induced either thermally or photochemically, as well as substitution and functionalization of the CO moiety. Importantly, the complexes exhibit strong Si–CO bonding, with CO→Si σ-donation and Si→CO π-backbonding, which is reminiscent of transition-metal carbonyls. This similarity between the abundant semi-metal silicon and rare transition metals may provide new opportunities for the development of silicon-based catalysis.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
X-ray crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under the reference numbers CCDC 1976834 (3) and CCDC 1976835 (5) via https://www.ccdc.cam.ac.uk/structures/. All other data supporting the findings are contained in the main text or the Supplementary Information.
References
Herrmann, W. A. 100 years of metal carbonyls: a serendipitous chemical discovery of major scientific and industrial impact. J. Organomet. Chem. 383, 21–44 (1990).
Werner, H. Complexes of carbon monoxide and its relatives: An organometallic family celebrates its birthday. Angew. Chem. Int. Ed. 29, 1077–1089 (1990).
Holleman, A. F., Wiberg, E. & Wiberg, N. Lehrbuch der Anorganischen Chemie Vol. 102 (de Gruyter, 2007).
Behrens, H. The chemistry of metal carbonyls: ‘the life work of Walter Hieber’. J. Organomet. Chem. 94, 139–159 (1975).
Beller, M. in Topics in Organometallic Chemistry Vol. 18 (Springer-Verlag, 2006).
Power, P. P. Main-group elements as transition metals. Nature 463, 171–177 (2010).
Martin, D., Soleilhavoup, M. & Bertrand, G. Stable singlet carbenes as mimics for transition metal centers. Chem. Sci. 2, 389–399 (2011).
Weetman, C. & Inoue, S. The road travelled: after main-group elements as transition metals. ChemCatChem 10, 4213–4228 (2018).
Légaré, M.-A., Pranckevicius, C. & Braunschweig, H. Metallomimetic chemistry of boron. Chem. Rev. 119, 8231–8261 (2019).
Braunschweig, H. et al. Multiple complexation of CO and related ligands to a main-group element. Nature 522, 327–330 (2015).
Puschmann, F. F. et al. Phosphination of carbon monoxide: A simple synthesis of sodium phosphaethynolate (NaOCP). Angew. Chem. Int. Ed. 50, 8420–8423 (2011).
Hansmann, M. M. & Bertrand, G. Transition-metal-like behavior of main group elements: ligand exchange at a phosphinidene. J. Am. Chem. Soc. 138, 15885–15888 (2016).
Wu, X. et al. Observation of alkaline earth complexes M(CO)8 (M = Ca, Sr, or Ba) that mimic transition metals. Science 361, 912–916 (2018).
Lavallo, V., Canac, Y., Donnadieu, B., Schoeller, W. W. & Bertrand, G. CO fixation to stable acyclic and cyclic alkyl amino carbenes: Stable amino ketenes with a small HOMO–LUMO gap. Angew. Chem. Int. Ed. 45, 3488–3491 (2006).
Hudnall, T. W. & Bielawski, C. W. An N,N′-diamidocarbene: studies in C–H insertion, reversible carbonylation, and transition-metal coordination chemistry. J. Am. Chem. Soc. 131, 16039–16041 (2009).
Lee, V. Y. Organosilicon Compounds: Theory and Experiment (Synthesis) Vol. 1 (Academic Press, 2017).
Pearsall, M. A. & West, R. The reactions of diorganosilylenes with carbon monoxide. J. Am. Chem. Soc. 110, 7228–7229 (1988).
Arrington, C. A., Petty, J. T., Payne, S. E. & Haskins, W. C. K. The reaction of dimethylsilylene with carbon monoxide in low-temperature matrices. J. Am. Chem. Soc. 110, 6240–6241 (1988).
Hamilton, T. P. & Schaefer, H. F. III Silaketene: A product of the reaction between silylene and carbon monoxide? J. Chem. Phys. 90, 1031–1035 (1989).
Tacke, M. et al. Complexes of decamethylsilicocene: Cp2*Si(CO) and Cp2*Si(N2). Z. Anorg. Allg. Chem. 619, 865–868 (1993).
Maier, G., Reisenauer, H. P. & Egenolf, H. Quest for silaketene: A matrix-spectroscopic and theoretical study. Organometallics 18, 2155–2161 (1999).
Becerra, R., Cannady, J. P. & Walsh, R. Silylene does react with carbon monoxide: some gas-phase kinetic and theoretical studies. J. Phys. Chem. A 105, 1897–1903 (2001).
Bornemann, H. & Sander, W. Reactions of methyl(phenyl)silylene with CO and PH3—the formation of acid–base complexes. J. Organomet. Chem. 641, 156–164 (2002).
Protchenko, A. V. et al. Reduction of carbon oxides by an acyclic silylene: reductive coupling of CO. Angew. Chem. Int. Ed. 58, 1808–1812 (2019).
Wang, Y. et al. Silicon-mediated selective homo- and heterocoupling of carbon monoxide. J. Am. Chem. Soc. 141, 626–634 (2019).
Xiong, Y., Yao, S., Szilvási, T., Ruzicka, A. & Driess, M. Homocoupling of CO and isocyanide mediated by a C,C′-bis(silylenyl)-substituted ortho-carborane. Chem. Commun. 56, 747–750 (2020).
Ganesamoorthy, C. et al. A silicon–carbonyl complex stable at room temperature. Nat. Chem. 12, 608–614 (2020).
Reiter, D. et al. Disilene-silylene interconversion: A synthetically accessible acyclic bis(silyl)silylene. J. Am. Chem. Soc. 141, 13536–13546 (2019).
Huber, K. P. & Herzberg, G. Molecular Spectra and Molecular Structure IV. Constants of Diatomic Molecules Vol. 4 (Van Nostrand Reinhold Company, 1979).
Fischer, R. C. & Power, P. P. π-Bonding and the lone pair effect in multiple bonds involving heavier main group elements: developments in the new millennium. Chem. Rev. 110, 3877–3923 (2010).
Bader, R. F. W. Atoms in Molecules: A Quantum Theory (Oxford Univ. Press, 1990).
Macchi, P. & Sironi, A. Chemical bonding in transition metal carbonyl clusters: complementary analysis of theoretical and experimental electron densities. Coord. Chem. Rev. 238–239, 383–412 (2003).
Macchi, P. & Sironi, A. in The Quantum Theory of Atoms in Molecules: From Solid State to DNA and Drug Design (eds Matta, C. F. & Boyd, R. J.) Ch. 13 (Wiley-VCH, Weinheim, 2007).
Schweizer, J. I. et al. A disilene base adduct with a dative Si–Si single bond. Angew. Chem. Int. Ed. 55, 1782–1786 (2016).
Stanford, M. W. et al. Intercepting the disilene-silylsilylene equilibrium. Angew. Chem. Int. Ed. 58, 1329–1333 (2019).
Cabeza, J. A., Van der Maelen, J. F. & García-Granda, S. Topological analysis of the electron density in the N-heterocyclic carbene triruthenium cluster [Ru3(μ-H)2(μ3-MeImCH)(CO)9] (Me2Im = 1,3-dimethylimidazol-2-ylidene). Organometallics 28, 3666–3672 (2009).
Wendel, D. et al. From Si(ii) to Si(iv) and back: Reversible intramolecular carbon–carbon bond activation by an acyclic iminosilylene. J. Am. Chem. Soc. 139, 8134–8137 (2017).
Wendel, D. et al. Silicon and oxygen’s bond of affection: an acyclic three-coordinate silanone and its transformation to an iminosiloxysilylene. J. Am. Chem. Soc. 139, 17193–17198 (2017).
Boyarskiy, V. P., Bokach, N. A., Luzyanin, K. V. & Kukushkin, V. Y. Metal-mediated and metal-catalyzed reactions of isocyanides. Chem. Rev. 115, 2698–2779 (2015).
Takeda, N., Kajiwara, T., Suzuki, H., Okazaki, R. & Tokitoh, N. Synthesis and properties of the first stable silylene–isocyanide complexes. Chem. Eur. J. 9, 3530–3543 (2003).
Abe, T., Iwamoto, T., Kabuto, C. & Kira, M. Synthesis, structure, and bonding of stable dialkylsilaketenimines. J. Am. Chem. Soc. 128, 4228–4229 (2006).
Mansikkamäki, A., Power, P. P. & Tuononen, H. M. Computational analysis of n→π* back-bonding in metallylene–isocyanide complexes R2MCNR′ (M = Si, Ge, Sn; R = tBu, Ph; R′ = Me, tBu, Ph). Organometallics 32, 6690–6700 (2013).
Beck, W. & Fehlhammer, W. P. Reactions of metal carbonyls with the azide ion and—vice versa—reactions of azido complexes with carbon monoxide: isocyanato complexes. analogous reactions in NO+/N3− transition metal chemistry. Z. Anorg. Allg. Chem. 636, 157–162 (2010).
Acknowledgements
We thank M. C. Holthausen and J. I. Schweizer for fruitful discussions, computational resources and advice. Quantum chemical calculations were performed in part at the Leibniz Supercomputing Center of the Bavarian Academy of Science and Humanities. We acknowledge M. Muhr (R. A. Fischer) for the LIFDI-MS measurement. We thank the WACKER Chemie AG and the European Research Council (SILION 637394) for continued financial support.
Author information
Authors and Affiliations
Contributions
D.R. and R.H. planned and carried out all experiments and analysed the data. A.P. designed and conducted the computational investigations. P.F. performed the SC-XRD measurements. S.I. designed and conceived the project. D.R. and S.I. wrote the manuscript with input and critical revision from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Discussion and Tables 1–27.
Supplementary Data 1
Cif file compound 3.
Supplementary Data 2
Cif file compound 5.
Supplementary Data
Cartesian coordinates for the optimized structures.
Rights and permissions
About this article
Cite this article
Reiter, D., Holzner, R., Porzelt, A. et al. Silylated silicon–carbonyl complexes as mimics of ubiquitous transition-metal carbonyls. Nat. Chem. 12, 1131–1135 (2020). https://doi.org/10.1038/s41557-020-00555-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41557-020-00555-4
This article is cited by
-
A class of non-aromatic 1,3-disilapyrroles acting as stable organosilicon-based triplet diradicals
Nature Synthesis (2023)
-
Synthesis, isolation and application of a sila-ketenyl anion
Nature Synthesis (2023)
-
Nonclassical complex of dichlorosilylene with CO: direct spectroscopic detection
Russian Chemical Bulletin (2021)
-
Main group carbonyl complexes
Communications Chemistry (2020)
-
Surprisingly stable Si–CO species
Nature Chemistry (2020)