Abstract
The dry reforming of methane provides an attractive route to convert greenhouse gases (CH4 and CO2) into valuable syngas, so as to resolve the carbon cycle and environmental issues. However, the development of high-performance catalysts remains a huge challenge. Herein, we report a 0.6% Ir/CeO2−x catalyst with a metal-support interface structure which exhibits high CH4 (~72%) and CO2 (~82%) conversion and a CH4 reaction rate of ~973 μmolCH4 gcat−1 s−1 which is stable over 100 h at 700 °C. The performance of the catalyst is close to the state-of-the-art in this area of research. A combination of in situ spectroscopic characterization and theoretical calculations highlight the importance of the interfacial structure as an intrinsic active center to facilitate the CH4 dissociation (the rate-determining step) and the CH2* oxidation to CH2O* without coke formation, which accounts for the long-term stability. The catalyst in this work has a potential application prospect in the field of high-value utilization of carbon resources.
Similar content being viewed by others
Introduction
Owing to the increasing global warming and climate change issues, strategies for greenhouse gas reduction have drawn extensive interest from both fundamental research and industrial applications1,2,3. CO2 and CH4 are regarded as two predominant contributors to the greenhouse effect; therefore, their utilization and conversion to high-value-added chemicals and fuels meet the demands for achieving large-scale carbon fixation, carbon emission reduction and carbon cycle4,5,6,7. One promising approach is to convert both CO2 and CH4 simultaneously through thermo-catalytic dry reforming of methane (DRM) reaction, which produces the syngas (H2 and CO) as an important platform for alternatives of petroleum-derived fuels and valuable chemicals8,9,10,11. Thermodynamically, the DRM reaction involves both C–H bond dissociation (439 kJ mol−1) and C=O bond hydrogenation (750 kJ mol−1) followed by subsequent formation of CO and H2, resulting in a highly endothermic process (ΔH298K = 247 kJ mol−1)12,13,14,15. This normally requires a high energy consumption and rigorous reaction temperature (>800 °C) to maintain favorable catalytic activity, but suffers from serious catalyst deactivation due to nanoparticle agglomeration and carbon deposition16,17,18. In this case, great efforts have been focused on the exploration of catalysts towards DRM reaction, such as supported noble metals (e.g., Pt19, Ru9,20, and Pd21,22) and non-noble metals (e.g., Ni23,24,25 and Co26) catalysts. Although considerable advances have been made, rational design and preparation of highly efficient catalysts to acquire high activity and stability simultaneously, still remain a big challenge.
In general, pure metal surfaces exhibit low reactivity towards methane dissociation and are prone to deactivation resulting from carbon deposition; whilst both experimental and theoretical studies have shown that C–H bond activation is more sensitive to coordinatively unsaturated metallic sites12,27. In this respect, the emerging strong metal-support interaction (SMSI) has demonstrated many appealing advantages, such as the interfacial structure and synergistic catalysis, which have attached widespread research interest in various heterogeneous reactions (e.g., CO2 methanation and water gas shift reaction)28,29,30,31. The fine-tuning for SMSI has been successfully employed to optimize geometric/electronic structure of metal species at the interface32,33,34, which provides great opportunities to promote catalytic performance towards DRM reaction. On the one hand, the oxidic Mδ+ metal species formed at the interfacial sites as an electron–acceptor, would reduce the Td symmetry structure of methane molecule and thus facilitate its activation dehydrogenation to CHx6,12,20. For instance, Pirovano et al. reported that Ni2+ species promotes C−H bond dissociation at a lower temperature relative to metal Ni based on experiments and DFT calculations35. On the other hand, reducible supports (e.g., CeO2, ZrO2, and TiO2), which renders a facile conversion between two oxidation states (e.g., Ce4+ and Ce3+), would stabilize oxidic Mδ+ species via accommodating metal-to-support electron transfer32,36,37,38. Meanwhile, the concomitant oxygen vacancies make a great contribution to elevate the activation adsorption of C=O group and facilitate the transformation of intermediates34,39,40. For example, Liu et al. reported the oxygen vacancies on CeO2 surface serve as active center towards CO2 hydrogenation to methanol, where the catalytic activity is highly correlated with the oxygen vacancies concentration41. This evokes us to design a suitable metal-support interface structure with synergistic catalysis effect, so as to simultaneously promote catalytic activity and stability for DRM reaction and further reveal the structure-property correlation at molecular/atomic scale.
Herein, we report an Ir nanoclusters supported on CeO2 catalyst prepared through a facile impregnation-reduction method. HAADF-STEM, quasi in situ XPS and in situ XAFS confirm the formation of interface structure (Irδ+−Ov−Ce3+), whose concentration can be modulated via adjusting the Ir loading. The optimal catalyst 0.6% Ir/CeO2−x (Fig. 1a) exhibits high conversions of CH4 (~72%) and CO2 (~82%) at 700 °C, with a CH4 reaction rate of ~973 μmolCH4 gcat−1 s−1; and a 100 h stream-on-line test demonstrates a satisfactory stability without obvious deactivation. This is, to the best of our knowledge, preponderant to the state-of-the-art catalysts under similar reaction conditions. Kinetics studies verify that the dissociation of CH4 is the rate-determining step in DRM reaction, whose activation energy decreases significantly by ~50 kJ mol−1 owing to the interfacial synergistic catalysis. Operando investigations (DRIFTS and XAFS), catalytic evaluations and DFT calculations substantiate that the interfacial sites (Irδ+−Ov−Ce3+) serve as the intrinsic active center: CH4 molecule undergoes activation adsorption and dissociation to CH2* species and H2 at the interfacial Irδ+ site, and then CH2* experiences oxidation by neighboring oxygen species to generate CH2O*, followed by CH2O* dehydrogenation to produce CO and H2; the concomitant Ov is replenished by the activation adsorption of C=O group in CO2. This interfacial synergistic catalysis not only enhances the catalytic activity for DRM reaction, but also inhibits catalyst deactivation from excessive decomposition of CH2* species to carbon deposition.
a Schematic illustration of Ir/CeO2−x samples. b, d TEM and HR-TEM images of 0.6% Ir/CeO2−x. c Particle size of various Ir/CeO2−x and Ir/Al2O3 samples determined by TEM. e, f High-resolution AC-HAADF-STEM images of 0.2% and 0.6% Ir/CeO2−x, respectively. g, h AC-HAADF-STEM image and corresponding EDS mapping of 0.6% Ir/CeO2−x. i Corresponding elemental line scanning of 0.6% Ir/CeO2−x.
Results and discussion
Structural characterizations of catalysts
Both Ir/Al2O3 and Ir/CeO2−x samples with various Ir loading (0.2%–3%) were prepared via a facile impregnation-reduction method, whose XRD patterns (Supplementary Fig. 1) displayed a series of characteristic reflections indexed to a typical Al2O3 (JCPDS 77-0396) and CeO2 (JCPDS 78-0694) phase, respectively. No recognizable diffractions peaks of Ir or IrO2 are found for these samples, implying a highly dispersed Ir species and/or a low Ir content. TEM images of Ir/CeO2−x samples (Fig. 1b) show numerous Ir nanoclusters are well dispersed and anchored onto the CeO2 nanorods support, in which the mean particle size of Ir increases from ~1.0 nm (0.2% and 0.6% Ir/CeO2−x) to ~1.5 nm (2% Ir/CeO2−x) and then to ~1.6 nm (3.0% Ir/CeO2−x) (Fig. 1c and Supplementary Fig. 4−7). Accordingly, the dispersion of Ir (DIr, Supplementary Table 1) decreases gradually from 82% (0.2% Ir/CeO2−x) to 39% (3.0% Ir/CeO2−x). From the local magnification HR-TEM images (Fig. 1d and Supplementary Fig. 3−7), two clear crystalline phases are identified as ~0.19 and ~0.31 nm, respectively, corresponding to the (110) and (111) planes of CeO2 nanorods support. As shown in Supplementary Fig. 8, the (110) facet is predominantly exposed accompanied with minor (111) facet, consistent with the previous reports41,42. As a control sample, 0.6% Ir/Al2O3 displays a larger particle size (~1.3 nm) and a lower dispersion (~65%) relative to 0.6% Ir/CeO2−x, along with lattice spacings of ~0.19 and ~0.23 nm ascribed to (400) and (311) planes of Al2O343. In addition, the aberration-correction high-angle annular dark-field scanning transmission electron microscopy (AC-HAADF-STEM) was conducted to explore detailed structure of Ir/CeO2−x. As shown in Fig. 1e and f, a clear lattice fringe (~0.211 nm) indexed to Ir(111) plane is observed on the surface of CeO2 for both the 0.2% and 0.6% Ir/CeO2−x samples. Moreover, the energy dispersive spectroscopy (EDS) elemental mapping and elemental line scanning of 0.6% Ir/CeO2−x sample (Fig. 1g−i) show a partial coating of CeO2 on the surface of Ir cluster, indicating the formation of interfacial structure between Ir and CeO2.
Quasi in situ XPS spectra were performed to investigate the electronic structure of surface Ir species. As shown in Fig. 2a, the Ir/Al2O3 sample displays two peaks at 60.6 eV (Ir 4f7/2) and 63.6 eV (Ir 4f5/2) corresponding to the Ir0 species. In contrast, for the four Ir/CeO2−x samples, besides the same Ir0 peaks, two additional strong peaks at 61.6 eV (Ir 4f7/2) and 64.6 eV (Ir 4f5/2) are found, which are attributed to the Irδ+ species44,45,46. This indicates the electron transfer from Ir species to CeO2 support at the interface via the electronic metal-support interaction (EMSI), which is absent in the Ir/Al2O3 sample. With the increase of Ir content, the ratio of Irδ+/(Irδ++Ir0) declines gradually from 57% (0.2% Ir/CeO2−x) to 30% (3.0% Ir/CeO2−x) (Supplementary Table 2), as a result of the decreased Ir dispersion degree. Furthermore, in situ CO-DRIFTS is implemented to investigate the configuration of Ir species (Fig. 2d), from which a broad band centered at ~2020 cm−1 due to the linear CO at Ir0 site is found for the Ir/Al2O3 sample. Notably, in the case of Ir/CeO2−x samples, both the linear adsorption of CO at Ir0 (~2020 cm−1) and gem-dicarbonyl species adsorption at Irδ+ (~2060 cm−1) are observed44,45,47. With the increment of Ir loading, according to the Gaussian peak fitting results, the relative peak intensity of Irδ+/(Irδ++Ir0) displays an obvious decrease from 0.2% Ir/CeO2−x (56%) to 3.0% Ir/CeO2−x (23%) (Supplementary Table 3), in accordance with the variation tendency of Irδ+/(Irδ++Ir0) in the XPS results.
a–c Quasi in situ XPS of Ir 4f, Ce 3d an O 1s for Ir/Al2O3 and Ir/CeO2−x samples with various Ir loading. d In situ CO-DRIFTS spectra on the surface over Ir/Al2O3 and various Ir/CeO2−x samples. e Ir L3-edge XANES spectra and (f) diagram of the linear combination fitting (LCF) results for various samples.
X-ray absorption near-edge structure (XANES) measurements at normalized Ir L3-edge were implemented to analyze the electronic state and coordination fine structure. As shown in Fig. 2e, the white line peaks of Ir/CeO2−x and Ir/Al2O3 samples are located between Ir foil and IrO2 reference, suggesting the existence of positively charged Ir species. Moreover, the intensity of white line declines gradually from 0.2% Ir/CeO2−x to 3.0% Ir/CeO2−x and then to Ir/Al2O3, indicating the decrease in the oxidation state of Irδ+ species (reduced interfacial electron transfer) along with the increase in metallic Ir0. The Fourier transforms of the extended X-ray absorption fine spectra (EXAFS) in the R space (Supplementary Fig. 10) show that all these samples exhibit coexistent of Ir−O scattering (~1.5 Å) and Ir−Ir scattering (~2.5 Å). Accordingly, we conducted the linear combination fitting (LCF) of XANES (Fig. 2f) to determine the Ir species composition in these samples. The control sample Ir/Al2O3 displays a low Ir4+ atomic ratio of 27%. In contrast, the Ir4+ is predominant for the Ir/CeO2−x samples, in which the Ir4+ atomic ratio of 0.2% and 0.6% Ir/CeO2−x samples are 63% and 59% (Supplementary Table 4), respectively; whilst the Ir0 plays a leading role for the 2.0% and 3.0% Ir/CeO2−x samples, as a result of the increased particle size of Ir. The average oxidation state of iridium species is calculated based on the results from LCF analysis, which gives the following sequence: 0.2% Ir/CeO2−x (+2.5) > 0.6% Ir/CeO2−x (+2.4) > 2.0% Ir/CeO2−x (+1.8) > 3.0% Ir/CeO2−x (+1.7) > 0.6% Ir/Al2O3 (+1.1).
Then, we used quasi in situ XPS to study the defective sites of Ir/CeO2−x samples, which contribute to mediate the CO2 activation. For the pristine CeO2 support (Supplementary Fig. 13), the spectra of Ce 3d show six strong peaks at 882.9, 889.0, 898.6 eV (marked as v, v″, and v‴, respectively) and 901.5, 907.6, 917.2 eV (marked as u, u″, and u‴, respectively) assigned to 3d104f 0 state of Ce4+ species, with a spin-orbit splitting of about 18.6 eV. In terms of Ir/CeO2−x samples (Fig. 2b), four peaks appear at 880.5 eV, 885.2 eV, 899.1 eV, and 903.8 eV (marked as v0, v′, u0, and u′, respectively) belonging to 3d104f 1 state of Ce3+ 41,48. The relative concentration of surface Ce3+, calculated by Ce3+/(Ce3+ + Ce4+) based on corresponding peak areas (Supplementary Table 2), decreases gradually from 23% (0.2% Ir/CeO2−x) to 10% (3.0% Ir/CeO2−x). Moreover, the oxygen vacancies of Ir/CeO2−x and Ir/Al2O3 samples were further analyzed via deconvolution of quasi in situ O 1s XPS spectra. As shown in Fig. 2c, three peaks are found at 529.3, 530.8 and above 532.8 eV, which are assigned to the lattice oxygen (Ol), oxygen vacancies (Ov) and other weakly bound oxygen species (Oc, such as hydroxyl oxygen or chemisorbed oxygen species), respectively48,49. The relative ratio of oxygen vacancy (Ov) calculated as Ov/(Ol + Ov + Oc) ranks in the following order: 0.2% Ir/CeO2−x (39%) > 0.6% Ir/CeO2−x (36%) > 2.0% Ir/CeO2−x (26%) > 3.0% Ir/CeO2−x (21%) (Supplementary Table 2), in agreement with the tendency of Ce3+/(Ce3+ + Ce4+) ratio. Therefore, in situ CO-DRIFTS, XAFS and quasi in situ XPS results notarize the formation of interface structure (Irδ+−Ov−Ce3+) originating from SMSI, whose relative concentration decreases with increment of Ir loading.
Catalytic evaluations
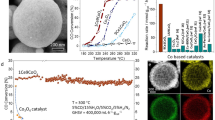
The preceding samples were evaluated for DRM under a gas hourly space velocity as high as 240000 mL g−1 h−1 at atmospheric pressure. As shown in Fig. 3a and b, both the CH4 and CO2 conversions as a function of reaction temperature show a positive correlation for these samples, duo to the strong endothermic characteristic. The control sample 0.6% Ir/Al2O3 gives a normal catalytic performance towards DRM reaction; whilst the catalytic performance of Ir/CeO2−x samples improve significantly. Notably, the catalytic activity exhibits a volcanic curve at each reaction temperature along with the increase of Ir loading: an increase from 0.2% Ir/CeO2−x to 0.6% Ir/CeO2−x (the maximum value) is present, followed by a slight descend to 2.0% Ir/CeO2−x and 3.0% Ir/CeO2−x. As for the optimal 0.6% Ir/CeO2−x sample, both the CH4 and CO2 conversions reach up to the thermodynamic equilibrium, and the reaction rate is 3−20 times higher than previously reported studies under similar reaction conditions within 650−750 °C (Fig. 3c and Supplementary Table 5)19,50. Specifically, the 0.6% Ir/CeO2−x catalyst exhibits high conversions of CH4 (72%) and CO2 (82%) with a CH4 reaction rate of ~973 μmolCH4 gcat−1 s−1 at a relatively moderate temperature (700 °C), which are precedent to the state-of-the-art catalysts6,7,9,16,20,24,25,49,50,51. In addition, the long-term stability test displays a rapid deactivation for the Ir/Al2O3 sample within 15 h due to Ir agglomeration and carbon deposition at 700 °C (Supplementary Fig. 16 and 17). In contrast, both the CH4 and CO2 conversions of 0.6% Ir/CeO2−x catalyst remain almost unchanged within 100 h on stream (Fig. 3d). Moreover, the used 0.6% Ir/CeO2−x catalyst does not show obvious structural change compared with the fresh sample, verified by TEM, XPS and in situ CO-DRIFTS (Supplementary Fig. 18−20), indicating a satisfactory stability in DRM reaction. The results above demonstrate excellent performance of 0.6% Ir/CeO2−x catalyst, which shows potential application in industrial applications.
a, b CH4 conversion and CO2 conversion over various samples at 650, 700, and 750 °C. c Comparison study on mass specific activity between 0.6% Ir/CeO2−x and other typical catalysts used in DRM reaction64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79. d Stability test of 0.6% Ir/CeO2−x and 0.6% Ir/Al2O3 at 700 °C for DRM. Evaluated conditions: CH4/CO2/N2 = 20/20/5 mL min−1, GHSV = 240000 mL g−1 h−1. e, f Correlation of CH4 or CO2 partial pressure on the reaction rates of CH4 and CO2. g Kinetic studies and calculated activation energy (Ea) of CH4 over various catalysts. h Intrinsic TOF over various catalysts within the catalytic dynamic range. i TOF as a function of interfacial Ir concentration calculated by CO-DRIFTS results.
Furthermore, we performed kinetic studies on CH4 and CO2 activation as well as the rate-determining step in DRM system. Firstly, the effects of external and internal diffusion limitation have been eliminated under the aforementioned reaction conditions34,50,52. On this basis, the kinetic experimental data were studied via setting a stationary partial pressure of one reactant whilst changing the other partial pressure (Fig. 3e, f), and the obtained results were calculated for kinetic parameters and were shown in Supplementary Table 6. The reaction rate over 0.6% Ir/CeO2−x catalyst displays a linear positive correlation with the partial pressure of CH4 and CO2. Nevertheless, the calculated reaction order with respect to CH4 (~0.67 and ~0.53) is significantly higher than that of CO2 (~0.09 and ~0.07), indicating that the CH4 activation is critical to the reaction kinetics, consistent with previous studies50,52,53. Moreover, the apparent activation energy (Ea) of CH4 over 0.6% Ir/CeO2−x is 91 kJ mol−1, much larger than that of CO2 (70 kJ mol−1) (Fig. 3g and Supplementary Fig. 22 and 23). The results verify that the CH4 dissociation entails a higher energy barrier and serves as the rate-determining step in this catalytic system. Notably, the Ea value on 0.6% Ir/CeO2−x catalyst shows a marked decline by 35% relative to the 0.6% Ir/Al2O3 sample, which indicates the interfacial sites play a critical role in activating reactant molecule. In addition, the intrinsic TOF of CH4 is evaluated at a low conversion (below 15%, Fig. 3h), which gives a decrease order as follows: 0.2% Ir/CeO2−x (168 molCH4 molIr−1 s−1) > 0.6% Ir/CeO2−x (163 molCH4 molIr−1 s−1) > 2.0% Ir/CeO2−x (122 molCH4 molIr−1 s−1) > 3.0% Ir/CeO2−x (110 molCH4 molIr−1 s−1). To further the reveal correlation of intrinsic active site and structure-property, the intrinsic TOF of CH4 is plotted as a function of interfacial Irδ+ concentration (based on the results of in situ CO-DRIFTS), from which an approximative linear relationship is present (Fig. 3i). Furthermore, a positive correlation between intrinsic TOF and surface Ce3+ ratio (Supplementary Fig. 24) or surface oxygen vacancy ratio (Supplementary Fig. 25) is also demonstrated. The results above elucidate that the Irδ+−Ov−Ce3+ interfacial sites serve as the intrinsic active center towards DRM reaction, accounting for the prominent catalytic performance.
Catalytic mechanism
In situ/operando XANES of Ir L3-edge and Ce L3-edge combined with quasi in situ XPS were applied to reveal the dynamic variation of fine structure and electronic interaction at interfacial sites under the catalytic reaction. During the measurement, CH4 and CO2 was introduced into the reaction cell in turn, and the catalytic reaction was triggered at 700 °C via injecting the second reactant gas, so as to observe the formation and variation of interface structure (Irδ+−Ov−Ce3+). When CH4 is introduced alone, the white line peaks of Ir and Ce shift close to the reference Ir foil and CeF3 (Fig. 4a, d), respectively, indicating a decline in valence states of Ir and Ce (Fig. 4b, e). The corresponding variations in XPS spectra of Ir 4f and Ce 3d are also observed (Fig. 4c, f): the Irδ+/(Irδ++Ir0) ratio decreases whilst the Ce3+/(Ce3+ + Ce4+) ratio and Ov increase. This implies the occurrence of CH4 dissociation to CHx* species, which then combines with surface reactive O to generate more Irδ+−Ov−Ce3+ interface sites. After the injection of CO2, the white line peaks of Ir and Ce shift back to their original position, indicating the replenishment of Ov by CO2. In Fig. 4a, d, as CO2 is introduced alone, the white line peaks of Ir and Ce move close to the reference IrO2 and CeO2, respectively (elimination of primary Ov); and XPS results show the increased valence states of Ir and Ce accompanied with reduced Ce3+/(Ce3+ + Ce4+) ratio and Ov (Fig. 4b, e). Afterwards, the subsequent CH4 flowing induces the recovery of Ir and Ce white line peaks to their original position, corresponding to the CH4 dissociation assisted with surface reactive oxygen species.
a, b In situ/operando normalized XANES at Ir L3-edge and diagram of the linear combination fitting (LCF) results of 0.6% Ir/CeO2−x with CH4, CO2 and CH4 + CO2 treatment, respectively. d, e Ce L3-edge and diagram of the linear combination fitting (LCF) results of 0.6% Ir/CeO2−x with CH4, CO2 and CH4 + CO2 treatment, respectively. c, f Quasi in situ XPS spectra of Ir 4f and Ce 3d for the fresh 0.6% Ir/CeO2−x and the same catalyst after CH4 or CO2 treatment at 700 °C.
In situ/operando DRIFTS experiments of reactants were carried out to further identify the intermediate species and monitor the evolution of dynamic reaction process at the interface structure Irδ+−Ov−Ce3+ (Fig. 5a−h). When CH4 is introduced individually into the reactor at 700 °C, in addition to the gas phase CH4 at ~3016 and ~1304 cm−1, another two bands at ~1330 and ~1350 cm−1 corresponding to the deformation vibration of CHx* and CH3* are observed4,20,51, respectively, due to the activation adsorption and dissociation of CH4 at interface Irδ+ sites. Subsequently, with the injection of CO2, two strong peaks located at ~2360 and ~1550 cm−1, as well as another broad one at ~3750−3550 cm−1 appear, which are attributed to the gas phase CO2, the monodentate carbonate species (HCOO*) and surface hydroxyl group (OH*), respectively (Fig. 5b−d and Supplementary Figs. 28, 29)16,20,54,55. Notably, another IR band assigned to the CHxO* species is found at ~1390 cm−1, accompanied with the weakened bands of CH4 and CHx* species56,57. This is probably due to the oxidation of CHx* by reactive oxygen species originating from CO2 dissociation. In addition, three bands between ~2200 and ~2000 cm−1 are detected, which are ascribed to gaseous CO and adsorbed CO* at Irδ+, respectively (Fig. 5c)44,45,46. Once the atmosphere is switched from the mixture gas (CO2 and CH4) to individual CH4, the bands of gas phase CO2 weaken firstly, and then the bands assigned to CHxO*, HCOO*, OH* and CO species disappear gradually accompanied with the enhancement of CH4 and CHx* peaks. This demonstrates the oxygen-containing species (CHxO*, HCOO*, OH*) serves as important intermediate, whose consumption can be reproduced by CO2 at interface Ov.
In situ/operando DRIFTS spectra over 0.6% Ir/CeO2−x at 700 °C after in-situ pretreatment and He purging, followed by exposure to: (a−d) first CH4 atmosphere, subsequent CH4 + CO2 and then CH4 atmosphere for 30 min, respectively; (e−h) first CO2 atmosphere, subsequent CO2 + CH4 and then CO2 atmosphere for 30 min, respectively. i Schematic illustration for DRM reaction at the interface of Ir/CeO2−x. Ir, green; Ce, yellow; C, gray; O, crimson; H, white. The inset shows potential energy profile of CH4 decomposition by Ir/CeO2−x(110). ‘TS’ represents a transition state. The black and orange numbers represent the adsorption energies and energy barriers of the elementary steps, respectively.
Next, we changed the study paradigm, in which CO2 is injected into the reactor firstly under the same conditions. Accordingly, the bands assigned to CO2 is observed (Supplementary Fig. 30 and Fig. 5e). With the subsequent flowing of CH4, the bands of CH4, CHx* and CH3* species are found (Fig. 5g, h), followed by the emergence of CHxO* and CO peaks as well as the weakened OH* band. This verifies the significance of CHxO* species originating from the reaction between CHx* and surface oxygen species, in accordance with the results of Fig. 5a−d. Operando investigations above (XAFS and DRIFTS) substantiate that the interface structure (Irδ+−Ov−Ce3+) serves as the intrinsic active center with a crucial synergistic effect: Irδ+ promotes the activation adsorption of CH4 molecule whilst CO2 dissociation occurs at the Ce3+−Ov site, followed by the formation of the key intermediate (CHxO* species).
To in-depth explore the decisive role of Irδ+−Ov−Ce3+ interfacial sites in the reaction process, DFT calculations were investigated on Ir7/CeO2−x model based on the experimental results (Supplementary Fig. 31). As shown in Fig. 5i and Supplementary Fig. 32, firstly, CH4 molecule undergoes adsorption at the interfacial Irδ+ of Ir7/CeO2−x (110) with a small adsorption energy (−0.03 eV); then, the C–H bond cleavage of CH4 occurs to generate CH3* (TS1: 1.12 eV). Afterwards, the CH3* species experiences dehydrogenation process which shows an energy barrier of 1.43 eV, excluding the oxidation of CH3* to CH3O* with a large steric hindrance. Subsequently, two possible steps are involved: (1) CH2* oxidation to CH2O* and (2) CH2* dehydrogenation to CH*. However, the former displays a much lower energy barrier (TS3: 1.03 eV) than the latter (TS4: 1.56 eV), in agreement with the formation of CHxO* intermediate verified by the operando DRIFTS results. This step is crucial, which inhibits excessive decomposition of CH2* species to carbon deposition. The next dehydrogenation of CH2O* to CHO* (TS5) and CO (TS6) shows normal activation barriers of 0.63 and 0.73 eV, respectively. Finally, the produced CO undergoes desorption from the Ov and the remaining four active hydrogen form into two H2 molecules (Supplementary Fig. 32). Meanwhile, CO2 molecule experiences dissociation adsorption at the Ov on the surface with an adsorption energy of −1.85 eV and an energy barrier of 0.7 eV (Supplementary Fig. 33 and 34), with the formation of active oxygen species that participates in the CH2* oxidation to CH2O*. According to the calculation results, the dehydrogenation of CH3* species to CH2* gives the highest energy barrier (1.43 eV), which is determined as the rate-determining step of DRM reaction, in accordance with the experimental results (Fig. 3e−f). In addition, a comparative study between Ir7/CeO2−x and Ir7/Al2O3 shows that the reaction energy barrier of rate-determiningstep in the former case (1.43 eV) is significantly lower than the latter one (Supplementary Figs. 35, 36: 2.88 eV), demonstrating the essential contributions of interface sites (Irδ+−Ov−Ce3+), in well agreement with the catalytic performance in Fig. 3a−h.
In summary, we report an Ir/CeO2−x catalytic system with metal–support interface structure towards DRM reaction. The obtained 0.6% Ir/CeO2−x catalyst exhibits exceptional conversions of CH4 (72%) and CO2 (82%), a CH4 reaction rate of ~973 μmolCH4 gcat−1 s−1 and a satisfactory service stability within 100 h at a relatively low temperature (700 °C). A joint investigation based on HAADF-STEM, quasi in situ XPS and in situ XAFS confirms the formation of interface structure (Irδ+−Ov−Ce3+), whose concentration can be modulated via adjusting the Ir loading. Operando investigations (DRIFTS and XAFS), catalytic evaluations and DFT calculations substantiate that the interfacial sites (Irδ+−Ov−Ce3+) serve as the intrinsic active center to facilitate the dissociation of CH4 (the rate-determining step) and the oxidation of CH2* to CH2O*; the concomitant Ov can be replenished by the activation adsorption of CO2. This interfacial synergistic catalysis plays a crucial role in boosting the catalytic performance and inhibiting deactivation, which paves a way for the design of other high-performance heterogeneous catalysts towards structure-sensitive reactions.
Methods
Chemicals and materials
Analytical grade chemical reagents were purchased in Aladdin company and used directly without further purification, including: Ce(NO3)3·6H2O, NaOH, Al2O3, and H2IrCl6·6H2O. Deionized water was adopted in all experiment steps.
Preparation of catalysts
CeO2 nanorods were prepared via a hydrothermal method reported by our group42. Typically, Ce(NO3)3 solution (0.4 M, 20 mL) and NaOH solution (6.8 M, 140 mL) were fully mixed with vigorous stirring for 30 min at room temperature. The obtained milky slurry was placed into a 200 mL sealed Teflon autoclave for 24 h at 100 °C. After filtering, washing thoroughly, and drying at 65 °C for 18 h, the sample was calcined in air at 500 °C with a heating rate of 10 °C min−1 for 4 h to obtain the CeO2 nanorods support. CeO2 (0.5 g) was dispersed into deionized water (20 ml) and H2IrCl6·6H2O aqueous solution (0.022 g mL−1; 0.105, 0.315, 1.050, 1.575 mL, respectively) was slowly dripped into above solution with vigorous stirring for various Ir loading samples. After 8 h of reaction, the resulting precipitate was centrifuged, washed thoroughly with deionized water and ethanol, followed by drying at 60 °C for 12 h. Before the DRM reaction, the sample was pre-treated at 750 °C for 3 h in a gaseous mixture of CH4 and CO2 (1:1, v/v; total flow rate: 50 mL min−1). As a reference, the Ir/Al2O3 sample was prepared via the same method described above by using Al2O3 as the support, in which the pre-treated steps are in accordance with those of Ir/CeO2−x samples.
Characterizations
X-ray diffraction (XRD) experiments were carried out with Bruker D8 Advance diffractometer. The elemental content was determined by Shimadzu ICPS-7500 equipment. The morphology and structure of catalysts were studied on JEOL JEM-2010 high-resolution transmission electron microscope. AC-HAADF-STEM images and EDS mapping data were performed on JEOL JEM-ARM200F. The CO pulses chemisorption experiments were conducted on Micromeritics Autochem II 2920. Quasi in situ XPS measurements were recorded on Kratos Axis Ultra DLD Instrument. The pre-treated sample was placed in a glove box and transferred into a sample rod in a N2 atmosphere. In situ/Operando XAFS at Ir L3-edge and Ce L3-edge measurements were recorded at the beamline BL11B of the Shanghai Synchrotron Radiation Facility (SSRF), Shanghai Institute of Applied Physics, Chinese Academy of Sciences (CAS). In situ/operando DRIFTS were studied on a Bruker TENSOR II infrared spectrometer with a MCT detector. The detailed experimental methods are present in the Supplementary Information.
DFT calculations
The density functional theory (DFT) calculations based on first-principle methodology were investigated using the Vienna ab initio simulation package (VASP 5.4.4)58,59. Generalized gradient approximation (GGA) of PBE functional was applied to describe the exchange and correlation energy. Grimme’s DFT-D3 method and projector augmented wave (PAW) method were employed to illustrate the effect of van der Waals interaction and to depict the core electrons, respectively60,61. The climbing image nudged elastic band (CI-NEB) method was employed to determine reaction transition states62,63.
Data availability
The primary data that support the plots within this paper and other finding of this study are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Zhao, H. et al. The role of Cu1–O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 5, 818–831 (2022).
Zito, A. M. et al. Electrochemical carbon dioxide capture and concentration. Chem. Rev. 123, 8069–8098 (2023).
Song, Y. et al. Dry reforming of methane by stable Ni–Mo nanocatalysts on single-crystalline MgO. Science 367, 777–781 (2020).
Xiao, Y. et al. Active exsolved metal-oxide interfaces in porous single‐crystalline ceria monoliths for efficient and durable CH4/CO2 reforming. Angew. Chem. Int. Ed. 61, e202113079 (2021).
Pakhare, D. et al. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 43, 7813–7837 (2014).
Akri, M. et al. Atomically dispersed nickel as coke-resistant active sites for methane dry reforming. Nat. Commun. 10, 5181 (2019).
Guo, D. et al. Enhanced thermocatalytic stability by coupling nickel step sites with nitrogen heteroatoms for dry reforming of methane. ACS Catal. 12, 316–330 (2021).
Zhu, Q. et al. Enhanced CO2 utilization in dry reforming of methane achieved through nickel-mediated hydrogen spillover in zeolite crystals. Nat. Catal. 5, 1030–1037 (2022).
Tang, Y. et al. Synergy of single-atom Ni1 and Ru1 sites on CeO2 for dry reforming of CH4. J. Am. Chem. Soc. 141, 7283–7293 (2019).
Zhang, X. et al. A novel Ni–MoCxOy interfacial catalyst for syngas production via the chemical looping dry reforming of methane. Chem 9, 102–116 (2023).
Joo, S. et al. Enhancing thermocatalytic activities by upshifting the d‐band center of exsolved Co−Ni−Fe ternary alloy nanoparticles for the dry reforming of methane. Angew. Chem. Int. Ed. 60, 15912–15919 (2021).
Schwach, P. et al. Direct conversion of methane to value-added chemicals over heterogeneous catalysts: challenges and prospects. Chem. Rev. 117, 8497–8520 (2017).
Meng, X. et al. Direct methane conversion under mild condition by thermo-, electro-, or photocatalysis. Chem 5, 2296–2325 (2019).
Fan, L. et al. Strategies in catalysts and electrolyzer design for electrochemical CO2 reduction toward C2+ products. Sci. Adv. 6, eaay3111 (2020).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Cheng, F. et al. Dry reforming of CH4/CO2 by stable Ni nanocrystals on porous single‐crystalline MgO monoliths at reduced temperature. Angew. Chem. Int. Ed. 60, 18792–18799 (2021).
Zhang, J.-C. et al. Robust ruthenium-saving catalyst for high-temperature carbon dioxide reforming of methane. ACS Catal. 10, 783–791 (2019).
Zhang, X. et al. High-performance binary Mo–Ni catalysts for efficient carbon removal during carbon dioxide reforming of methane. ACS Catal. 11, 12087–12095 (2021).
Niu, J. et al. Unraveling enhanced activity, selectivity, and coke resistance of Pt–Ni bimetallic clusters in dry reforming. ACS Catal. 11, 2398–2411 (2021).
Liu, Z. et al. Highly active ceria-supported Ru catalyst for the dry reforming of methane: in situ identification of Ruδ+–Ce3+ interactions for enhanced conversion. ACS Catal. 9, 3349–3359 (2019).
Danielis, M. et al. Outstanding methane oxidation performance of palladium-embedded ceria catalysts prepared by a one-step dry ball-milling method. Angew. Chem. Int. Ed. 57, 10212–10216 (2018).
Köpfle, N. et al. Zirconium-assisted activation of palladium to boost syngas production by methane dry reforming. Angew. Chem. Int. Ed. 57, 14613–14618 (2018).
Tavasoli, A. et al. Enhanced hybrid photocatalytic dry reforming using a phosphated Ni−CeO2 nanorod heterostructure. Nat. Commun. 14, 1435 (2023).
Palmer, C. et al. Dry reforming of methane catalysed by molten metal alloys. Nat. Catal. 3, 83–89 (2020).
Kim, S. M. et al. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 139, 1937–1949 (2017).
Wu, Z. et al. Lattice srained Ni-Co alloy as a high-performance catalyst for catalytic dry reforming of methane. ACS Catal. 9, 2693–2700 (2019).
Wei, J. et al. Structural and mechanistic requirements for methane activation and chemical conversion on supported iridium clusters. Angew. Chem. Int. Ed. 116, 3771–3774 (2004).
Li, S. et al. Tuning the selectivity of catalytic carbon dioxide hydrogenation over iridium/cerium oxide catalysts with a strong metal-support interaction. Angew. Chem. Int. Ed. 56, 10761–10765 (2017).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018).
Xu, M. et al. Insights into interfacial synergistic catalysis over Ni@TiO2–x catalyst toward water–gas shift reaction. J. Am. Chem. Soc. 140, 11241–11251 (2018).
Pu, T. et al. Engineering heterogeneous catalysis with strong metal–support interactions: characterization, theory and manipulation. Angew. Chem. Int. Ed. 62, e202212278 (2022).
Chen, A. et al. Structure of the catalytically active copper–ceria interfacial perimeter. Nat. Catal. 2, 334–341 (2019).
Parastaev, A. et al. Breaking structure sensitivity in CO2 hydrogenation by tuning metal–oxide interfaces in supported cobalt nanoparticles. Nat. Catal. 5, 1051–1060 (2022).
Meng, H. et al. A strong bimetal-support interaction in ethanol steam reforming. Nat. Commun. 14, 3189 (2023).
Lustemberg, P. G. et al. Room-temperature activation of methane and dry re-forming with CO2 on Ni-CeO2(111) surfaces: effect of Ce3+ sites and metal–support interactions on C–H bond cleavage. ACS Catal. 6, 8184–8191 (2016).
Maurer, F. et al. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 3, 824–833 (2020).
Parastaev, A. et al. Boosting CO2 hydrogenation via size-dependent metal–support interactions in cobalt/ceria-based catalysts. Nat. Catal. 3, 526–533 (2020).
Pinheiro Araújo, T. et al. Flame-made ternary Pd−In2O3−ZrO2 catalyst with enhanced oxygen vacancy generation for CO2 hydrogenation to methanol. Nat. Commun. 13, 5610 (2022).
Wang, F. et al. Active site dependent reaction mechanism over Ru/CeO2 catalyst toward CO2 methanation. J. Am. Chem. Soc. 138, 6298–6305 (2016).
Wu, C. Y. et al. Inverse ZrO2/Cu as a highly efficient methanol synthesis catalyst from CO2 hydrogenation. Nat. Commun. 11, 5767 (2020).
Jiang, F. et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 10, 11493–11509 (2020).
Wang, F. et al. Catalytic behavior of supported Ru nanoparticles on the {100}, {110}, and {111} facet of CeO2. J. Catal. 329, 177–186 (2015).
Meng, H. et al. Highly efficient hydrogen production from dehydrogenation reaction of nitrogen heterocycles via Pd0–Pdδ+ synergistic catalysis. ACS Catal. 13, 9234–9244 (2023).
Zhang, Y. et al. Tuning selectivity of CO2 hydrogenation by modulating the strong metal–support interaction over Ir/TiO2 catalysts. Green. Chem. 22, 6855–6861 (2020).
Jin, R. et al. Low temperature oxidation of ethane to oxygenates by oxygen over iridium-cluster catalysts. J. Am. Chem. Soc. 141, 18921–18925 (2019).
Ye, X. et al. Highly selective hydrogenation of CO2 to ethanol via designed bifunctional Ir1−In2O3 single-atom catalyst. J. Am. Chem. Soc. 142, 19001–19005 (2020).
Lu, Y. et al. A versatile approach for quantification of surface site fractions using reaction kinetics: The case of CO oxidation on supported Ir single atoms and nanoparticles. J. Catal. 378, 121–130 (2019).
Li, J. et al. Distribution and valence state of Ru species on CeO2 supports: support shape effect and its influence on CO oxidation. ACS Catal. 9, 11088–11103 (2019).
Safavinia, B. et al. Enhancing CexZr1–xO2 activity for methane dry reforming using subsurface Ni dopants. ACS Catal. 10, 4070–4079 (2020).
Wang, J. et al. Design of a carbon-resistant Ni@S−2 reforming catalyst: controllable Ni nanoparticles sandwiched in a peasecod-like structure. Appl. Catal. B: Environ. 282, 119546 (2021).
Kim, S. et al. Yolk–shell Pt-NiCe@SiO2 single-atom-alloy catalysts for low-temperature dry reforming of methane. ACS Catal. 11, 8247–8260 (2021).
Yan, X. et al. Highly efficient and stable Ni/CeO2−SiO2 catalyst for dry reforming of methane: effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B: Environ. 246, 221–231 (2019).
Li, H. et al. Ultra-durable Ni-Ir/MgAl2O4 catalysts for dry reforming of methane enabled by dynamic balance between carbon deposition and elimination. Chem. Catal. 2, 1748–1763 (2022).
Das, S. et al. Effect of partial Fe substitution in La0.9Sr0.1NiO3 perovskite-derived catalysts on the reaction mechanism of methane dry reforming. ACS Catal. 10, 12466–12486 (2020).
Xie, Y. et al. Frustrated lewis pairs boosting low-temperature CO2 methanation performance over Ni/CeO2 nanocatalysts. ACS Catal. 12, 10587–10602 (2022).
Bu, K. et al. Methane dry reforming over boron nitride interface-confined and LDHs-derived Ni catalysts. Appl. Catal. B: Environ. 252, 86–97 (2019).
Shen, D. et al. Synergistic Pt−CeO2 interface boosting low temperature dry reforming of methane. Appl. Catal. B: Environ. 318, 121809 (2022).
Kresse, G. et al. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. et al. Efficient iterative schemes forab initiototal-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter Mater. Phys. 54, 11169–11186 (1996).
Kresse, G. et al. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B: Condens. Matter Mater. Phys. 59, 1758–1775 (1999).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B: Condens. Matter Mater. Phys. 50, 17953–17979 (1994).
Yang, J. et al. A DFT+U study of acetylene selective hydrogenation on oxygen defective anatase (101) and rutile (110) TiO2 supported Pd4 cluster. J. Chem. Phys. 136, 104107 (2012).
Heyden, A. et al. Efficient methods for finding transition states in chemical reactions: Comparison of improved dimer method and partitioned rational function optimization method. J. Chem. Phys. 123, 224101 (2005).
Tsoukalou, A. et al. Dry-reforming of methane over bimetallic Ni−M/La2O3 (M = Co, Fe): the effect of the rate of La2O2CO3 formation and phase stability on the catalytic activity and stability. J. Catal. 343, 208–214 (2016).
Li, K. et al. Dry reforming of methane over La2O2CO3-modified Ni/Al2O3 catalysts with moderate metal support interaction. Appl. Catal. B: Environ. 264, 118448 (2020).
García-Diéguez, M. et al. Characterization of alumina-supported Pt, Ni and PtNi alloy catalysts for the dry reforming of methane. J. Catal. 274, 11–20 (2010).
Dou, J. et al. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B: Environ. 254, 612–623 (2019).
Zhou, H. et al. A single source method to generate Ru−Ni−MgO catalysts for methane dry reforming and the kinetic effect of Ru on carbon deposition and gasification. Appl. Catal. B: Environ. 233, 143–159 (2018).
Naeem, M. A. et al. Exsolution of metallic Ru nanoparticles from fefective, fluorite-type solid solutions Sm2RuxCe2–xO7 to impart stability on dry reforming catalysts. ACS Catal. 10, 1923–1937 (2019).
Wu, H. et al. Bi- and trimetallic Ni catalysts over Al2O3 and Al2O3−MO (M = Ce or Mg) oxides for methane dry reforming: Au and Pt additive effects. Appl. Catal. B: Environ. 156−157, 350–361 (2014).
Li, D. et al. Carbon dioxide reforming of methane over Ru catalysts supported on Mg−Al oxides: a highly dispersed and stable Ru/Mg(Al)O catalyst. Appl. Catal. B: Environ. 200, 566–577 (2017).
Dong, J. et al. Reaction-induced strong metal–support interactions between metals and inert boron nitride nanosheets. J. Am. Chem. Soc. 142, 17167–17174 (2020).
Qian, L. et al. The promotion effect of hydrogen spillover on CH4 reforming with CO2 over Rh/MCF catalysts. Appl. Catal. B: Environ. 164, 168–175 (2015).
Yentekakis, I. V. et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B: Environ. 243, 490–501 (2019).
Wang, F. et al. Tuning the metal-support interaction in catalysts for highly efficient methane dry reforming reaction. Appl. Catal. B: Environ. 180, 511–520 (2016).
Whang, H. S. et al. Enhanced activity and durability of Ru catalyst dispersed on zirconia for dry reforming of methane. Catal. Today 293−294, 122–128 (2017).
Araiza, D. G. et al. Dry reforming of methane over Pt−Ni/CeO2 catalysts: effect of the metal composition on the stability. Catal. Today 360, 46–54 (2021).
da Fonseca, R. O. et al. Study of the effect of Gd-doping ceria on the performance of Pt/GdCeO2/Al2O3 catalysts for the dry reforming of methane. Catal. Today 355, 737–745 (2020).
Tathod, A. P. et al. Mediating interaction strength between nickel and zirconia using a mixed oxide nanosheets interlayer for methane dry reforming. Appl. Catal. B: Environ. 249, 106–115 (2019).
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFC2103500), the National Natural Science Foundation of China (22172006, 22102006, 22288102, and 22109177), and the Young Elite Scientists Sponsorship Program by CAST (2023QNRC001). The authors are thankful for the support of the SSRF (Shanghai Synchrotron Radiation Facility) during the XAFS measurements at the beamline of BL11B.
Author information
Authors and Affiliations
Contributions
M.W., Y.Y., G.C. and H.W. conceived the idea and designed the research. H.Y. and H.L. performed the DFT calculations. H.W., Z.L. and H.M. synthesized the catalysts and conducted the characterizations and reaction tests. L.W. and J.L. helped the STEM and XAFS analysis. All the authors analyzed the data and wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Petar Djinović and the other, anonymous, reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Cui, G., Lu, H. et al. Facilitating the dry reforming of methane with interfacial synergistic catalysis in an Ir@CeO2−x catalyst. Nat Commun 15, 3765 (2024). https://doi.org/10.1038/s41467-024-48122-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-48122-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.