Abstract
The direct oxidation of methane to methanol under mild conditions is challenging owing to its inadequate activity and low selectivity. A key objective is improving the selective oxidation of the first carbon-hydrogen bond of methane, while inhibiting the oxidation of the remaining carbon-hydrogen bonds to ensure high yield and selectivity of methanol. Here we design ultrathin PdxAuy nanosheets and revealed a volcano-type relationship between the binding strength of hydroxyl radical on the catalyst surface and catalytic performance using experimental and density functional theory results. Our investigations indicate a trade-off relationship between the reaction-triggering and reaction-conversion steps in the reaction process. The optimized Pd3Au1 nanosheets exhibits a methanol production rate of 147.8 millimoles per gram of Pd per hour, with a selectivity of 98% at 70 °C, representing one of the most efficient catalysts for the direct oxidation of methane to methanol.
Similar content being viewed by others
Introduction
Methane is a promising, clean, and cost-effective feedstock for producing high-value chemicals, such as methanol, which is a versatile energy carrier and platform molecule for the synthesis of important bulk chemicals like olefins and aromatics1,2. However, cleaving the first C–H bond in CH4 is difficult because of its high bond energy (439.3 kJ mol−1) and large ionization potential energy (13.0 eV)3. In addition, the CH3OH selectivity is uncontrollable because the remaining C-H bonds can be easily oxidized, leading to the overoxidation of CH4 to carbon dioxide (CO2)4. The conventional method for indirect conversion of CH4 to CH3OH involves the CH4 reforming to syngas (H2/CO) and subsequent synthesis of CH3OH using syngas as feedstock5. However, this energy-intensive method does not satisfy the requirements of green chemistry6,7. To address these limitations, the direct oxidation of CH4 to CH3OH (DOMM) under mild conditions was proposed1. This process is referred to as the “holy grail” reaction, and has been at the forefront of academic and industrial research for many decades8,9.
Recently, researchers proposed that precious metals can serve as promising catalysts for DOMM because they can effectively reduce the energy barriers and improve the reaction kinetics of C–H bond activation in aqueous media at mild temperatures (<80 °C). To date, the highest performance (91.8 mmol g−1 h−1) and selectivity (92%) of DOMM have been reported for the class of bimetallic PdAu alloy catalysts10. However, previous studies on PdAu alloy mainly focus on zero-dimensional (0D) nanoparticles, which suffer from hard-to-control structure regulations11,12 and random atom arrangements13; Consequently, establishing clear structure-activity relationships of PdAu catalysts and evaluating the roles of Pd and Au atoms are remarkably difficult. In addition, DOMM with H2O2 as the oxidant is a free-radical process, in which hydroxyl radicals (•OH) triggering the breakage of the first C–H bond is vitally important. However, insufficient attention has been devoted to the formation and/or triggering step of •OH14. Ultrathin two-dimensional (2D) nanostructures are superior to 0D nanoparticles in terms of their uniformly exposed facets and an ultrahigh fraction of surface atoms15,16. Additionally, the extended surface of 2D nanostructures represents an ideal research platform for regulating the performance of corresponding alloys and exploring the reaction mechanism of DOMM.
Herein, we regulated the coverage of Au atoms on ultrathin PdxAuy nanosheets (PdxAuy NS) using a facile galvanic replacement method and discovered a volcano-type performance–structure relationship between DOMM performance and Au atom coverage. Particularly, the optimized Pd3Au1 NS achieved a CH3OH production rate of 147.8 mmol g−1 h−1 with a high selectivity of 98% at 70 °C. Density functional theory (DFT) calculations suggested that the volcano-type relationship was governed by the energy barriers of the reaction-triggering and reaction-conversion steps on the surface of the PdxAuy NS. Moreover, the strength of the M–O bond measured by using the Integrated Crystal Orbital Hamilton Population (ICOHP) method was used as a promising catalytic descriptor (M–O ICOHP) because it was highly correlated with the energy barrier of the reaction-triggering and reaction-conversion steps. Therefore, the reason for the enhanced DOMM performance on PdxAuy NS was elucidated through the volcano-type relationship between the M–O ICOHP and catalytic performance.
Results
Synthesis and characterizations of PdxAuy NS
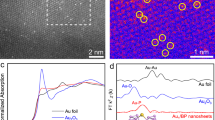
Following a typical synthesis process, the synthesized hexagonal Pd NS was used as seeds to obtain ultrathin PdxAuy NS with different coverage of Au atoms (details are presented in Supporting Information, Fig. 1a, Supplementary Fig. 1 and Table 1). The atomic ratios of Pd/Au were determined by inductively coupled plasma-optical emission spectrometry (ICP-OES) and denoted Pd33Au1 NS, Pd6Au1 NS, Pd3Au1 NS, and Pd1Au1 NS (Supplementary Fig. 2 and Table 2). The average diameters and lengths of the Pd NS were 60 and 30 nm, respectively (Fig. 1b and Supplementary Fig. 3). The special aberration-corrected scanning transmission electron microscopy (AC-STEM) images confirmed the high crystallinity of the Pd NS at the atomic scale, which had a lattice spacing of 0.224 nm for the face-centered cubic Pd (111) surface (Fig. 1c). The average thickness of each Pd NS was approximately 1.5 nm corresponding to seven layers of Pd atoms (Supplementary Fig. 4), being consistent with the atomic force microscopy results (Supplementary Fig. 5a). The hexagonal morphology of the PdxAuy NS was well preserved after replacing Pd atoms with Au atoms; however, a slight increase in their thickness was observed owing to the larger radius of Au atoms (Supplementary Figs. 5b, 6), and Pd1Au1 NS was eventually transformed into a Pd@Au core-shell structure (Supplementary Fig. 7). As shown in Fig. 1d, the Au atoms (bright dots) exclusively displaced the Pd atoms instead of simply depositing on the Pd NS surface. The incorporation of such few Au atoms had a negligible effect on the lattice spacing of the crystal face of Pd (111) (Fig. 1e). Pd6Au1 NS maintained the consummate lattice plane of Pd (111) with a larger lattice spacing (0.232 nm), and more Au single atoms and clusters were dispersed around Pd atoms (Fig. 1f, g). For Pd3Au1 NS with a lattice spacing of 0.240 nm, Au and Pd atoms (bright and dark dots in Fig. 1h, respectively) were uniformly arranged on the surface. The high-angle annular dark field (HAADF) STEM images and corresponding atomically resolved elemental mapping images showed an ordered atomic arrangement and a regular geometry profile (Fig. 1i). As shown in the X-ray diffraction (XRD) patterns of PdxAuy NS, the diffraction peaks shifted to a lower angle as the coverage of Au atoms increased, except for Pd1Au1 NS with core-shell structure. This result indicated that the lattice spacing was positively dependent on the Pd/Au ratio, in agreement with the AC-STEM results (Supplementary Fig. 8)17.
a Synthesis schematic of ultrathin PdxAuy NS. b Low magnification TEM image of Pd NS. Atomic-resolution AC-STEM images of (c) Pd NS, d, e Pd33Au1 NS, f, g Pd6Au1 NS, h Pd3Au1 NS. All insert images represent the selected fast Fourier transform images. i Atomically resolved elemental mapping images of Pd3Au1 NS.
The atomic electronic structure and coordination information of the PdxAuy NS were investigated using X-ray photoelectron spectroscopy (XPS) and synchrotron X-ray absorption spectroscopy. As shown in Fig. 2a, the peaks of Pd NS at 335.92 and 341.18 eV were attributed to 3d5/2 and 3d3/2 of metal Pd0, respectively18. In addition, the detected Pd2+ species, corresponding to peaks of 336.9 eV (3d5/2) and 342.66 eV (3d3/2) of Pd NS19,20 were attributed to slight oxidation of Pd atoms because of the exposure in air. With the increasing Au content, the Pd 3d peak of PdxAuy NS exhibited a higher binding energy than that of Pd NS, except for the Pd1Au1 NS with the core-shell structure, indicating the electron-deficient state of Pd atoms in PdxAuy NS (Supplementary Table 3). For the Au NS, the binding energy of 84.34 and 88.01 eV were attributed to 4 f7/2 and 4 f5/2 of metal Au0 (Fig. 2b). The Au 4f peaks of PdxAuy NS shifted to the lower binding energy compared with Au NS, and the value increased with increasing the Pd/Au ratio, indicating the significant electronic effects that the electron donation from Pd atoms to Au atoms, which was positively correlated with the coverage of Au atoms (Supplementary Table 4).
a XPS spectra of Pd 3d for Pd NS and PdxAuy NS. b XPS spectra of Au 4 f for Au NS and PdxAuy NS. c, d The K2-weighted and Fourier-transformed magnitudes of EXAFS spectra of the Pd K-edge and Au L3-edge. e Plots of intermetallic bond length versus Pd/Au ratio. f Plots of the coordination number of connected metals versus Pd/Au ratio.
In addition, the diffuse reflectance infrared Fourier transform spectroscopy using CO as a probe molecule (CO-DRIFTS) was also performed to assess the electronic state of the Au or Pd of PdxAuy NS (Supplementary Fig. 9a). The in-situ CO-DRIFTS spectra showed the physisorption peaks of CO molecule at 2171.7 and 2115.7 cm−1 were gradually eliminated over time by sweeping with 10 vol% He/Ar, and the bridged chemisorption peak of CO molecule at 1859.7 and 1915.1 cm−1 were present on the surfaces of Pd and Pd3Au1 NS, respectively (Supplementary Figs. 9b, c). Compared with Pd and Au NS, the chemisorption peaks of PdxAuy NS exhibited a significant blueshift with increasing Au coverage (Supplementary Fig. 9d)21,22,23, indicating the decrease of electron cloud density around Pd atoms, consistent with the XPS results.
The valence state of PdxAuy NS was further explored by X-ray absorption near edge structure (XANES) spectroscopy. The energy of the inflection point (ΔE) is the shift in the absorption edge of the sample with respect to that of a standard foil24. The higher ΔE of the Pd K-edge signals for PdxAuy NS was an indication of slight oxidation of Pd atoms (Supplementary Fig. 10a), whereas the lower valence state of the Au atoms was confirmed by the decreased ΔE (Supplementary Fig. 10b). The XANES results further confirmed the electronic interactions between Pd and Au atoms in PdxAuy NS24,25,26,27,28. The atomic coordination information of PdxAuy NS near the Pd K-edge (Fig. 2c) and Au L3-edge (Fig. 2d) was shown in the k4-weighted and Fourier transform extended X-ray absorption fine structure (EXAFS) spectra. There were no signals indicating the Au–Pd bond, mainly due to the small bond distances of Pd–Pd, Au–Au, and Pd–Au25,26. The shifts in both Pd–Pd and Au–Au bonds originate from the strong interactions between Au and Pd atoms, where the Au–Au bond exhibited a considerably higher shift25. In addition, the Pd–Pd and Pd–Au bond distances decreased as the coverage of Au atoms increased, whereas the Au–Au bond distance increased gradually due to the assembly of Au atoms (Fig. 2e and Supplementary Fig. 11). Furthermore, as the Au coverage increased, the coordination number of Pd–Pd (CN(Pd-Pd)) gradually decreased, whereas the Pd–Au (CN(Pd-Au)) increased, suggesting that the Pd atoms were replaced by Au atoms (Fig. 2f and Supplementary Fig. 12). Moreover, the increasing CN(Au-Au) and decreasing CN(Au-Pd), following the increasing coverage of Au atoms, suggested the accumulation of more Au atoms around each Pd atom and the formation of small Au clusters by the assembly of a few Au atoms. Therefore, the observed dependence of the bond distance and coordination number on the coverage of Au atoms demonstrated that the strong electronic interactions were present between Pd and Au atoms in the PdxAuy NS (Supplementary Table 5).
Performance of direct CH4 conversion
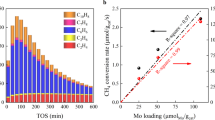
The catalytic performance of PdxAuy NS was evaluated for DOMM in a pressurized reactor (Supplementary Figs. 13 and 14). Gas chromatography and proton nuclear magnetic resonance (1H NMR) spectroscopy were employed to quantify the gaseous and liquid products (Supplementary Fig. 15). A CH3OH yield of 72.8 mmol g−1 h−1 with a selectivity of 96% was obtained with Pd NS as the catalyst (Fig. 3a)29,30. With increasing the coverage of Au atoms, a volcano-type performance could be observed, and a maximum yield of 147.8 mmol g−1 h−1 and selectivity of 98% was obtained with the Pd3Au1 NS4,31. Although Pd atoms were shown to be the primary active sites for DOMM, the enhanced performance of the PdxAuy NS catalyst originated from the Pd atoms affected by catalytically inactive Au atoms (Supplementary Fig. 16 and Supplementary Table 6). Further, the in-situ generation of H2O2 from O2 and H2 rather than the addition of H2O2 or H2O2/O2 as the oxidant was essential for enhancing the performance of DOMM (Supplementary Table 7)32,33. The turnover frequencies (TOFs) were determined to evaluate the intrinsic activity of the catalysts, and Pd3Au1 NS exhibited the highest TOF of all the samples. (Supplementary Table 8). More importantly, the yield and selectivity of Pd3Au1 NS did not decrease over 10 cycles, suggesting its high-performance stability (Fig. 3b). Furthermore, there was no change in the morphology and structure of Pd3Au1 NS after the 10-cycle test, confirming its structural stability (Supplementary Fig. 17). As shown in Fig. 3c, the performance of the Pd3Au1 NS was superior to those of previously reported catalysts under similar normalization conditions (Supplementary Table 9). Additionally, a CH3OH yield of 43.7 mmol g−1 h−1 and a selectivity of 95% were obtained for the Pd3Au1 NS at room temperature, suggesting its considerable potential for industrial application (Supplementary Table 9)2,34,35. The effects of various reaction conditions on the Pd3Au1 NS were systematically investigated. The yield and selectivity of the CH3OH product had a positive correlation with proper temperature and reaction time. (Fig. 3d, e and Supplementary Tables 10 and 11), and a proper pressure ratio of CH4/(H2 + O2) was considered to be important to regulate the amount of the in-situ generated H2O2 (Fig. 3f, Supplementary Table 12).
a The catalytic performance of DOMM for Pd, PdxAuy, and Au NS. b Reaction tests for the recycling and regeneration of the Pd3Au1 catalyst. c Comparison of catalytic performance and CH3OH selectivity for CH4 direct conversion with various catalysts (Supplementary Table 9). d The catalytic performance with different reaction temperatures for Pd3Au1 NS. e The catalytic performance with different reaction time at 70 °C for Pd3Au1 NS. f The catalytic performance with different CH4 vol. Pd3Au1 NS. All other conditions remain the same: 10 mL of water, 1 mg of catalyst, feed gas at 3.0 MPa with 1.1% H2/2.2% O2/67.2% CH4/20.57% Ar/8.93 % He. Each reaction was tested three times to obtain the error bars.
In situ characterization toward mechanism
Subsequently, the reaction mechanism of DOMM with the in-situ generation of H2O2 was analyzed. Time-resolved in-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was used to monitor the dynamics of the reaction intermediates on the surface of Pd3Au1 NS (Fig. 4a)36,37. The strong peaks at 3014 and 1303 cm−1 were attributed to the antisymmetric stretching νas(C–H) and bending δ(C–H) signal of adsorbed *CH4, respectively (Supplementary Fig. 18)38. The peaks at 1450 and 1342 cm−1 represented the shear and symmetric shaking vibration of adsorbed *CH3, respectively39. The broad peaks appearing in the range of 3200–2700 cm−1 and the peak at 1650 cm−1 were ascribed to the O–H stretching and bending δ(OH) signal, respectively39,40. In addition, the peak at 1136 cm−1 was attributed to the stretching vibration of *OCH3 derived from CH3OH. The small peaks between 2300 and 2400 cm−1, assigned to the antisymmetric stretching vibration of the adsorbed *CO2, indicated that the overoxidation of CH4 to CO239. Upon increasing the reaction time from 0.01 to 1.00 h, both the intensities of the O–H vibration peak and *CH3 peak increased, indicating that the activation of the first C–H bond to form the adsorbed *CH3 species was accomplished by the formation of *OH derived from the dissociation of the in-situ generated of H2O2. In addition, the signal intensity of *OCH3 gradually increased with increasing the reaction time representing the formation of more CH3OH product. To investigate the presence of free radicals, electron paramagnetic resonance (EPR) spectroscopy was conducted using 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) as a spin trap, with the Fenton reaction (Fe2+ + H2O2) for comparison14,41. As shown in Fig. 4b, the line (DMPO + H2O2) represented only the signal peaks of •OH free radical, and the line (DMPO + CH3OH + H2O2) suggested the signal peaks of •OH, •OOH, and •CH3 free radicals. Compared with these two lines, the ERP signals of Pd3Au1 NS presented the coexistence of •OH, •OOH, and •CH3 free radicals during the DOMM process. These results indicated that •OH radical derived from in-situ generated H2O2 could trigger the activation of C-H bond to form •CH3 radical, and the remaining •OH and •OOH radicals could combine •CH3 radical to form CH3OH and CH3OOH products, which was in accordance with DRIFTS results39. Subsequently, the relationship between the reaction time and product formation was evaluated using 1H NMR. As shown in Supplementary Fig. 19, CH3OOH was observed at 3.7 ppm in the early stages41,42, which was the product of the reaction between •OOH and •CH3 radicals. And CH3OH was probably obtained from the combination of •CH3 and •OH radicals and the decomposition of CH3OOH.
a In-situ DRIFTS spectra of adsorbed CH4, O2 and H2 at 70 °C for Pd3Au1 NS in the range of 3700 to 800 cm−1. The signal of EPR spectrum of (b) radical species (•CH3, •OH, •OOH). c CH4-TPD-MS results of Pd, Pd3Au1 and Au NS. d PdxAuy NS-dependency of in-situ generation H2O2 productivity for 70 °C in O2 and H2 atmosphere. Each reaction was tested three times to obtain the error bars.
Temperature-programmed desorption-mass spectrometry (TPD-MS) was used to measure the chemical adsorption strength of the adsorbent species on the catalyst surface18. The absence of the desorption peak on Au NS was attributed to the extremely low adsorption capacity for the CH4 molecule (Fig. 4c), while Pd NS exhibited a dominant desorption peak at 386 °C. The lower desorption intensity and temperature of Pd3Au1 NS implied that a moderate adsorption capacity for the CH4 molecule favored to the DOMM. In addition, thermal programmed desorption–mass spectroscopy (TPD-MS) proved the adsorption of CH4 and CH3 species on Pd3Au1 NS, which suggested that Pd3Au1 NS could activate the first C–H bond of CH4 without any oxidants at high temperature (Supplementary Fig. 20). The in-situ generation capacity of H2O2 for PdxAuy NS was examined using titanium oxalate spectrophotometry (Fig. 4d). A clear volcano-type relationship between H2O2 content and Pd/Au ratio was observed, and Pd3Au1 NS exhibits the highest H2O2 production rate of 2.4 mol g−1 h−1, which is also superior to those of the reported state-of-the-art catalysts (Supplementary Table 13). What’s more, the concentration of H2O2 remained at a high level with increasing reaction time (Supplementary Fig. 21), implying that more free radicals (•OH or •OOH) could remain on the surface of Pd3Au1 NS. Furthermore, the enhanced capacity of in-situ generation of H2O2 was explored by H2/O2-TPD measurements. Compared to Pd NS, Pd3Au1 NS exhibited weaker desorption peaks for H2 and O2 molecules, suggesting that the presence of Au atoms weakened the strong interaction between Pd atom and adsorbed O atom, preventing the O–O band breaking, to form the key intermediate of •OOH (Supplementary Figs. 22 and 23)43,44,45.
Theoretical calculations
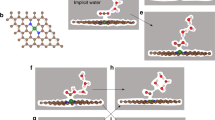
DFT calculations were conducted to reveal the origin of the volcano-type structure–performance relationship of PdxAuy NS. Four models with different Pd/Au ratios, namely, pure Pd, Pd2Au1, Pd1Au2, and pure Au skin, were adopted (Fig. 5a and Supplementary Fig. 24). As shown in Fig. 5b, efficient DOMM required sufficient •OH radicals to trigger the reaction and a low energy barrier to allow for rapid CH4 conversion to CH3OH10,11. Based on the experimental results, the reaction processes of DOMM on the surface of PdxAuy NS involved three key steps, namely, the formation step of the •OH radical (KS-1), activation step of the C–H bond of CH4 (KS-2), and formation step of the CH3OH product (KS-3), where KS-1was corresponded to the reaction-triggering step, and KS-2 and KS-3 were corresponded to the reaction-conversion step. The energy barriers of KS-1, KS-2, and KS-3 were denoted Ea1, Ea2, and Ea3, respectively. DFT calculations indicated that the introduction of Au into Pd NS significantly affected the in-situ generation of H2O2 from H2 and O2 (Supplementary Fig. 25). The monotonic increase in Ea1 with increasing the coverage of Au atoms confirmed that the introduction of Au could hinder the formation of •OH radicals, which was detrimental to the reaction-triggering step (Fig. 5c and Supplementary Fig. 26). In addition, the presence of Au atoms did not favor KS-2, as indicated by the larger Ea2; while it significantly facilitated KS-3, as suggested by the decreased Ea3 (Supplementary Fig. 27). Combining KS-2 and KS-3, the reaction-conversion step was assessed by the apparent reaction energy barrier (Eapp), defined as the energy difference between the highest energy barrier and initial reaction configuration (Supplementary Fig. 28). The Eapp exhibited a monotonical decrease from 1.21 to 0.96 eV with increasing the coverage of Au atoms (Fig. 5d and Supplementary Table 14). Among the four models, Pd skin with the lowest Ea1 exhibited the highest activity for the reaction-triggering step, while Au skin effectively facilitated the reaction-conversion step owing to the lowest Eapp. Therefore, for an effective DOMM process, both the reaction-triggering and reaction-conversion steps needed to be considered, and there was a trade-off between the two steps to achieve optimal performance.
a Structural model of four PdxAuy NS. b The total reaction pathway of the CH4 oxidation to CH3OH involves the in-situ generation of H2O2. The relationship of the coverage of Au atoms dotted Pd surface to c the energy barrier of H2O2 decomposition, d apparent reaction energy barrier, and e reaction rate indicator χ. f The dependence behavior between reaction rate indicator χ and M-O bond strength.
To characterize the aforementioned trade-off effect for the total DOMM process, the reaction rate indicator (χ), defined as χ = Ea1 + Eapp, was proposed based on the Arrhenius equation (details are presented in Supplementary Information). Remarkably, when the reaction-triggering and reaction-conversion steps were considered together, a volcano-type relationship between χ and the coverage of Au atoms could be clearly observed (Fig. 5e), being consistent with the experimental results (Fig. 3a). Therefore, χ could be used to evaluate the DOMM activity of PdxAuy NS. Pd2Au1 skin, with the lowest χ of 1.21 eV, was located at the volcanic peak and estimated to exhibit the best DOMM performance. The performance of the Pd skin (on the left) was limited by the conversion step with a larger Eapp, while the Au-rich surface (on the right) was limited by the triggering step because of the higher Ea1.
The microscopic mechanisms dominating the volcano-type relationship were further investigated. During the DOMM process, methyl and hydroxyl groups were chemisorbed on the catalyst surface to form M-C and M-O bonds (M represents metal), respectively. From pure Pd skin to pure Au skin, the ability of the PdxAuy NS to adsorb reaction intermediates decreased gradually because Au was more inert than Pd, characterized by the surface binding strength to methyl or hydroxyl groups. The binding strength, evaluated by the ICOHP, exhibited a monotonic decrease from Pd skin to Au skin (Supplementary Fig. 29 and Table 14). A more negative value of the ICOHP indicated a higher adsorption capacity of the catalyst surface for the reaction intermediates. Given that the •OH radical was both involved in the triggering and conversion steps, and M-O ICOHP was more sensitive to the Pd/Au ratios, thus the M-O ICOHP was applied to analyze the microscopic mechanism of the DOMM. A strong adsorption capacity was needed to facilitate the decomposition of H2O2 into •OH radicals during the reaction-triggering step (Supplementary Fig. 30a), while a weak adsorption capacity was favorable for the breaking of the M–O bond during the conversion step (Supplementary Fig. 30b). Therefore, a volcano-type relationship between χ and M–O binding strength was established by the trade-off effects (Fig. 5f). Note that this discussion was also applicative to the M-C ICOHP (Supplementary Fig. 31). For catalysts that bind OH groups too strong (M-O ICOHP < −2.95), the performance of DOMM was limited by the slow step of CH4 conversion to CH3OH. By contrast, for catalysts that bind OH groups too weak (M-O ICOHP > −2.95), the performance was limited by insufficient •OH radicals. As DFT calculations suggested, Pd2Au1 skin with an optimal M-O ICOHP of −2.95 would exhibit the best DOMM performance among the PdxAuy NS. This prediction was confirmed by the experimentally results that Pd3Au1 NS exhibited the best DOMM performance, with an atomic ratio close to that of Pd2Au1 skin (see the discussion below the Supplementary Fig. 24). In addition, the PdxAuy skin was thermodynamically unfavorable for further oxidation of •CH3 to methylene species, endowing a high selectivity for CH3OH (Supplementary Fig. 32).
Discussion
In this study, we investigated the coverage of Au atoms on PdxAuy NS and established a clear volcano-type structure–performance relationship between PdxAuy NS and their DOMM performance. The maximum yield and selectivity of Pd3Au1 NS for DOMM were 147.8 mmol g−1 h−1 and 98.0%, respectively. DFT calculations revealed that two steps, namely, the reaction-triggering and conversion step should be simultaneously considered for an effective DOMM process. The corresponding volcano-type relationship between the DOMM performance and M-O ICOHP suggested that M-O ICOHP could be regarded as a promising catalytic descriptor for this “holy grail” reaction, which could be an effective approach to balance the trade-off effect between the triggering and conversion steps and optimize the performance of PdxAuy catalysts. This study offers not only valuable insights into the reaction mechanisms of DOMM on PdAu alloys, but also a reliable model for developing such alloys with efficient performance.
Methods
Materials
Pd(acac)2 (Pd 34.9%, Macklin) and HAuCl4·3H2O (99.9%, Aladdin), Chloro(triphenylphosphine)gold(I) (AuPPh3Cl, 99%, Aladdin), N, N-dimethylformamide (DMF, 99.7%, Macklin), Tetrabutylammonium bromide (TBAB, 99%, Aldrich), Potassium titanium oxalate (C4H2K2OTi, 99%, Macklin), 1, 2-dichloropropane (99%, Macklin), 4-tert-butylpyridine (99%, Macklin), Poly(vinylpyrrolidone) (PVP, ~ 29000, Aldrich), Oleylamine (OM, 70%, Aldrich) and L-ascorbic acid (AA, 99.5%, Aldrich) and Carbon blacks (xc-72c, Macklin) were used. High-purity water (H2O, 18.3 MΩ cm) was employed for all experiments.
Preparation of Pd nanosheets
Typically, 185 mg TBAB, 50.0 mg Pd(acac)2, and 160.0 mg PVP were added into 12 mL DMF. The resulting solution was pipetted into a 50 mL glass flask. The flask was then pressurized with CO to 2 bar and kept at 80 °C for 3 h. By centrifugation at 12xg for 1.5 h, the colloidal Pd-blue nanosheets (NS) were precipitated. Finally, the obtained Pd NS were redispersed into 5 mL DMF for further use. The mass yields of the obtained Pd NS were ca. 90–95%.
Preparation of PdxAuy nanosheets
First, the synthesized Pd nanosheets (NS) and AuPPh3Cl (3 mg·mL−1 in DMF) were premixed into DMF to obtain a certain molar ratio of Pd:Au (Supplementary Table 1). Hydrazine (N2H4·H2O, 300 μL, 0.1 mM) was then added drop by drop. Once all the above steps had been completed, the solution was allowed to stand unattended at 25°C for 12 h. The products were collected via centrifuging. Finally, the obtained PdxAuy NS were redispersed into 10 mL H2O for subsequent applications. The mass yields of the obtained PdxAuy NS were ca. 90–95%.
Synthesis of Au nanosheets
Typically, 76.2 mL of hexane, 13.2 mL of OM, 1.8 mL of 1, 2-dichloropropane, 0.6 mL of 4-tert-butylpyridine and 30 mL of squalene were well vortexed in a 100 mL glass bottle for 0.08 h. Then this solution was rapidly added to a glass flask with 130 mg HAuCl4·3H2O while continuing to vortex and shake well. The mixed solution was placed in an oven preheated at 58 °C. The product was precipitated after 17 h by centrifugation (5xg, 0.05 h), and then washed three times with hexane and resuspended in hexane. The mass yields of the obtained Au NS were ca. 80–90%.
Characterization
The TEM and HAADF-STEM mapping images were characterized by three types of TEM instrument (thermoscientific Talos F200X G2; Titan G2 80-200 Chemi-STEM, FEI; and ARM200F, JEOL) operated at 200 kV. X-ray absorption fine structure (XAFS) spectra at Pd K-edge and Au L3-edge were performed at BL14W1 station (Shanghai, 3.5 GeV, and 250 mA). XPS was performed using a Shimadzu Axis Supra (Al Ka and hν = 1486.6 eV). XRD patterns were performed on a Rigaku Smart-Lab operating (Cu Ka, λ = 1.5406 Å, 40 kV, and 40 mA). The Pd and Au loading amounts were determined by the ICP-OES instrument. The tested CH3OH and CH3OOH (C1 liquid products) were prepared by adding 300 μL of electrolyte with 250 μL of D2O and 25 μL of DMSO solution (6 mM). The 1H spectrum peak of DMSO is at ~2.6 ppm. The 1H spectrum peak of D2O is at ~4.7 ppm. 1H spectrum peaks of CH3OH and CH3OOH are at ~3.3 and ~3.6 ppm, respectively. The total amount (CH3OH and CH3OOH) was gas chromatography (GC) analyzed, and the amount of CH3OOH was determined by the minus method. The CO2 was analyzed by GC with FID (Thermo Fisher, T1300). The standard curve method was employed to quantify the content of all products. The following formulae (1) and (2) were employed to calculate the CH3OH yield and selectivity of all products.
Catalytic methane conversion
Methane (CH4) conversion was conducted in a 50 mL autoclave with 1 mg catalyst with 10 wt% Pd3Au1 supported on carbon blacks. The chamber underwent three times of sealing and flushing using a gas mixture comprising of H2/O2/CH4/Ar/He (3.3%, 6.6%, 1.6%, 61.7%, and 26.8% by volume) and maintained at a pressure of 1.5 MPa. The mixture was agitated at 1.2xg and heated (1.5 °C /min) gradually to a specified temperature (e.g. 70 °C). Then, the autoclave was filled into CH4 gas at the pressure of 3.0 MPa, which continued to keep the target temperature with a regulated reaction time (e.g. 0.5 h). The autoclave was chilled to a temperature below 10 °C in ice in order to minimize the loss of volatile products at the conclusion of the reaction. After each reaction cycle, the catalyst was separated using centrifugation to investigate reusability. The catalyst was used in the next round after drying under vacuum at 80 °C for 12 h.
Turnover frequency calculation
The Turnover Frequency (TOF) numbers were calculated based following equation:
Where nCH3OH is the amount of substance of CH3OH, nsurface is the amount of substance of surface Pd atoms of Pd NS or PdxAuy NS. nmetal is the amount of substance of total Pd atoms of Pd NS or PdxAuy NS. mcat. is the mass of the catalyst. W is the mass loading of Pd NS or PdxAuy NS on carbon support, which is measured by using ICP-OES measurement. M is the atomic mass of Pd. T is the reaction time. δ is the molar percentage of the surface Pd atom of Pd NS or PdxAuy NS.
Calculation of the molar percentage of surface Pd atom (δ)
▓
For one Pd NS, ni is the amount of substance of surface Pd atom, and nj is the amount of substance of total Pd atom. N is the number of surface atoms. S is the one Pd NS surface area. Ssingle Pd atom is single Pd atom surface area. The density, volume, edge length, and thickness are ρ, V, t, and h. Where ρ = 12.02 g/cm3, t = 3 × 10−6 cm, h = 1.5 × 10−7 cm, V = 2.6 × t2 × h = 3.51 × 10−18 cm3, S = 2.61 × 10−11 cm2, Ssingle Pd atom = 1.3 × 10−15 cm2, M = 106.4 g/mol, NA = 6.02 × 10−23 mol−1, nj = (ρ × V)/M = 4.2 × 10−17 mol. Thus δPd of Pd NS was calculated as 8.4%. For δPdxAuy = mPdx × δ, mPdx is the atomic percentage of Pd in PdxAuy NS.
In-situ diffuse reflectance infrared Fourier transform spectroscopy measurements
In-situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) was used to investigate the dynamic evolution of adsorption species on the catalyst surface. PdxAuy NS were pretreated by heating at 100 °C under Ar flow (20 mL/min) for 1.0 h, and chilled at 70 °C under Ar. Then the gas mixture (H2/O2/CH4/Ar/He = 1.1%/2.2%/67.2%/20.57%/8.93%, v/v) was introduced. DRIFTS spectra were collected with 64 scans of the range 650–4000 cm−1 at a resolution of 4 cm−1.
In the CO-DRIFTS experiment, the PdxAuy NS was firstly treated in-situ by flowing a 10 vol% He/Ar mixture at 70 °C for 0.5 h. After that, the PdxAuy NS was chilled to 25 °C followed by introducing a 10% CO/Ar mixture at 40 mL/min. Spectra were recorded continuously until the CO adsorption signal reached a constant value. Finally, the gas flow was switched to 10 vol% He/Ar to remove any physically adsorbed species and surface chemisorption spectra were obtained.
Electron paramagnetic resonance test
Using a Bruker A320 Electron paramagnetic resonance (EPR) spectrometer (1MG (0.1UT)) and 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) as the scavenger, the free radicals produced during the direct oxidation of CH4 were detected. The DMPO-H2O solution was mixed with 1 mL of the reaction mixture (100 mmol L−1), and immediately transferred to a capillary tube (0.1 mm in diameter with a liquid fill height of approximately 5 cm). The distinctive peaks in the spectrometer’s resonant cavity were then used to identify the type of free radical. For instance, experiment 1 (DMPO + H2O2 + Fe2+) was composed of FeSO4·7H2O and HNO3 solution (1 mL, 50 mmol L−1, and pH = 4) combined with DMPO solution (1 mL, 100 mmol L−1).
Methane thermal programmed desorption test
Samples were gradually heated in a vacuum chamber, and the desorption process occurred when enough energy was available to overcome the desorption activation barrier of the species. After the adsorbates were removed from the catalyst surface in the gas form, they were analyzed by the mass spectrometer. Details are as follows:
The sample was weighed with 50 mg and dried from 25 °C to 150 °C at a rate of 10 °C/min. Ar gas (50 mL/min) was used to purify the sample for 1 h, after which the sample was chilled to 50 °C. Then, 50 mL of CH4/Ar (5%/95%, v/v) mixture was added at the same temperature for 0.5 h. Ar gas stream was replaced for 0.5 h to remove weakly bound CH4 from the surface. The gas was identified by using TCD in an Ar environment up to 700 °C (5 °C/min).
O2 thermal programmed desorption test
To begin with, 100 mg of catalyst was placed into a reaction tube and heated slowly to 150 °C for the purpose of dry pretreatment, followed by purging with He gas (40 mL/min) for 1 h and cooling to 50 °C. Subsequently, 40 mL of a O2/He (10%/90%, v/v) mixture was introduced to reach saturation for 1 h, and the He stream was then utilized to purge for 1 h to eliminate O2 with weak physical adsorption. Finally, the gas was identified using TCD in a He atmosphere up to 700 °C (10 °C/min).
H2 thermal programmed desorption test
The reaction tube was weighed with 100 mg of catalyst, which was dried at 150 °C (10 °C/min). After that, He gas (50 mL/min) was purged for 1 h. Following this, the temperature was cooled to 50 °C with a 10% H2/Ar mixture until saturation. Subsequently, the Ar gas stream was introduced with 50 mL/min for 1 h to eliminate any H2 with weak physical adsorption. Lastly, the surface was heated to 700 °C (15 °C/min) in an Ar environment and the desorbed gas was collected using TCD.
Density functional theory calculation
The VASP was utilized to perform spin-polarized DFT calculations, using the PBE functional and the PAW potential. A 500 eV energy cutoff and 10−5 eV convergence criterion were chosen for self-consistent calculations. For the purpose of the study, four models were constructed, based on a Pd (111) slab with a supercell (4 × 4 × 1) and 4 metal layers, including Pd skin, Pd2Au1 skin, Pd1Au2 skin, and Au skin. The vacuum layer between adjacent slab models had a thickness of approximately 15 Å and all structures were completely relaxed until the total force on each atom was below 0.05 eV/ Å. For sample the first Brillouin zone, a Γ-centered k-point mesh supercell was employed, while the DFT-D3 scheme was utilized to correct for van der Waals interactions. The COHP analysis was executed using the pbeVaspFit2015 base set in the LOBSTER code. The basis functions of 1 s, 2s2p, 2s2p, 4d5s, and 5d6s were H, C, O, Pd, and Au, respectively. The CI-NEB method was used to determine the energy barriers. To account for the solvent effect, the implicit solvation model via the VASPsol code. Computations were pre- and post-processed with VASPKIT code and VESTA software.
Reaction rate indicator (χ)
According to the Arrhenius equation, there was an exponential relationship between the reaction energy barrier (Ea) and the reaction rate (R). The exponential relationship could be expressed as R = A×exp[–Ea/(kBT)], where A, kB, and T were the pre-exponential factors that are considered as a constant, Boltzmann constant, and temperature. Direct CH4 oxidation to CH3OH involved the reaction triggering step and reaction conversion step, where the former was governed by H2O2 decomposition barrier (denoted as Ea1) and the latter was governed by the apparent reaction barrier (denoted as Eapp). Thus, the total reaction rate of the CH4 conversion to CH3OH could be described by the product of the Arrhenius relationship of these two processes: RCH4→CH3OH = A × exp[–Ea1/(kBT)] × exp[–Eapp/(kBT)]. This equation could be simplified as RCH4→CH3OH = A×exp[–(Ea1 + Eapp)/(kBT)]. The value of RCH4→CH3OH increased monotonically as (Ea1 + Eapp) increased. Hence, we proposed that χ could regarded as the reaction rate indicator on behalf of (Ea1 + Eapp).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request. Source data are provided in this paper. Source data are provided with this paper.
References
Gesser, H. D., Hunter, N. R. & Prakash, C. B. The Direct Conversion of Methane to Methanol by Controlled Oxidation. Chem. Rev. 85, 235–244 (1985).
Schwach, P., Pan, X. & Bao, X. Direct Conversion of Methane to Value-Added Chemicals over Heterogeneous Catalysts: Challenges and Prospects. Chem. Rev. 117, 8497–8520 (2017).
Meng, X. et al. Direct Methane Conversion under Mild Condition by Thermo-, Electro-, or Photocatalysis. Chem 5, 2296–2325 (2019).
Freakley, S. J. et al. Methane Oxidation to Methanol in Water. Acc. Chem. Res. 54, 2614–2623 (2021).
Liu, Y., Deng, D. & Bao, X. Catalysis for Selected C1 Chemistry. Chem 6, 2497–2514 (2020).
Jang, J., Shen, K. & Morales-Guio, C. G. Electrochemical Direct Partial Oxidation of Methane to Methanol. Joule 3, 2589–2593 (2019).
Luk, H. T., Mondelli, C., Ferré, D. C., Stewart, J. A. & Pérez-Ramírez, J. Status and Prospects in Higher Alcohols Synthesis from Syngas. Chem. Soc. Rev. 46, 1358–1426 (2017).
Gunsalus, N. J. et al. Homogeneous Functionalization of Methane. Chem. Rev. 117, 8521–8573 (2017).
Yan, Y. et al. High H2O2 Utilization Promotes Selective Oxidation of Methane to Methanol at Low Temperature. Front. Chem. 8, 252 (2020).
Jin, Z. et al. Hydrophobic Zeolite Modification for in Situ Peroxide Formation in Methane Oxidation to methanol. Science 367, 193–196 (2020).
Agarwal, N. et al. Aqueous Au-Pd Colloids Catalyze Selective CH4 Oxidation to CH3OH with O2 under Mild Conditions. Science 358, 223–227 (2017).
Edwards, J. K. et al. Direct Synthesis of H2O2 from H2 and O2 over Gold, Palladium, and Gold-Palladium Catalysts Supported on Acid-Pretreated TiO2. Angew. Chem., Int. Ed. 48, 8512–8515 (2009).
Ab Rahim, M. H. et al. Oxidation of Methane to Methanol with Hydrogen Peroxide Using Supported Gold-Palladium Alloy Nanoparticles. Angew. Chem., Int. Ed. 52, 1280–1284 (2013).
Luo, L. et al. Water Enables Mild Oxidation of Methane to Methanol on Gold Single-Atom Catalysts. Nat. Commun. 12, 1218 (2021).
Luo, M. et al. PdMo Bimetallene for Oxygen Reduction Catalysis. Nature 574, 81–85 (2019).
Wang, L. et al. Tunable Intrinsic Strain in Two-dimensional Transition Metal Electrocatalysts. Science 363, 870–874 (2019).
Wang, Y. et al. Ensemble Effect in Bimetallic Electrocatalysts for CO2. Reduction. J. Am. Chem. Soc. 141, 16635–16642 (2019).
Xu, Y. et al. Au Decorated Pd Nanowires for Methane Oxidation to Liquid C1 Products. Appl. Catal. B: Environ 308, 121223 (2022).
Liu, R. et al. Atomic-Level-Designed Catalytically Active Palladium Atoms on Ultrathin Gold Nanowires. Adv. Mater. 29, 1604571 (2017).
Dong, C. et al. Fully Exposed Palladium Cluster Catalysts Enable Hydrogen Production from Nitrogen Heterocycles. Nat. Catal. 5, 485–493 (2022).
Cattaneo, S. et al. Cinnamaldehyde hydrogenation using Au-Pd catalysts prepared by sol immobilisation. Catal. Sci. Technol. 8, 1677–1685 (2018).
Brehm, J., et al. Enhancing the Chemo-Enzymatic One-Pot Oxidation of Cyclohexane via In Situ H2O2 Production over Supported Pd-Based Catalysts. ACS Catal. 11776−11789 (2023).
Wang, Z., et al. Tailoring Electronic Properties and Atom Utilizations of the Pd Species Supported on Anatase TiO2{101} for Efficient CO2 Hydrogenation to Formic Acid. ACS Catal. 10056−10064 (2023).
Zhang, J. et al. Strong Metal–Support Interaction Boosts Activity, Selectivity, and Stability in Electrosynthesis of H2O2. J. Am. Chem. Soc. 144, 2255–2263 (2022).
Zhu, X. et al. Optimising Surface d Charge of AuPd Nanoalloy Catalysts for Enhanced Catalytic Activity. Nat. Commun. 10, 1428 (2019).
Ricciardulli, T. et al. Effect of Pd Coordination and Isolation on the Catalytic Reduction of O2 to H2O2 over PdAu Bimetallic Nanoparticles. J. Am. Chem. Soc. 143, 5445–5464 (2021).
Teng, X. et al. Formation of Pd/Au Nanostructures from Pd Nanowires. via Galvanic Replacement Reaction. J. Am. Chem. Soc. 130, 1093–1101 (2008).
Chen, C.-H. et al. Architecture of Pd–Au Bimetallic Nanoparticles in Sodium Bis(2-ethylhexyl)sulfosuccinate Reverse Micelles As Investigated by X-ray Absorption Spectroscopy. ACS Nano 1, 114–125 (2007).
Bai, S. X., Yao, Q., Xu, Y., Cao, K. L. & Huang, X. Q. Strong Synergy in a Lichen-like RuCu Nanosheet Boosts the Direct Methane Oxidation to Methanol. Nano Energy 71, 104566 (2020).
Jiang, W. et al. Pd-Modified ZnO–Au Enabling Alkoxy Intermediates Formation and Dehydrogenation for Photocatalytic Conversion. of Methane to Ethylene. J. Am. Chem. Soc. 143, 269–278 (2021).
Ni, F. et al. Selective Oxidation of Methane to Methanol via In Situ H2O2 Synthesis. ACS Org. Inorg. Au 3, 177–183 (2023).
Serra-Maia, R., Michel, F. M., Kang, Y. & Stach, E. A. Decomposition of Hydrogen Peroxide Catalyzed by AuPd Nanocatalysts during Methane Oxidation to Methanol. ACS Catal 10, 5115–5123 (2020).
Ab Rahim, M. H. et al. Low Temperature Selective Oxidation of Methane to Methanol Using Titania Supported Gold Palladium Copper Catalysts. Catal. Sci. Technol. 6, 3410–3418 (2016).
Cui, X. et al. Room-Temperature Methane Conversion by Graphene-Confined Single Iron Atoms. Chem 4, 1902–1910 (2018).
Hu, D.; Addad, A.; Ben Tayeb, K.; Ordomsky, V. V.; Khodakov, A. Y., Thermocatalysis enables photocatalytic oxidation of methane to formic acid at room temperature beyond the selectivity limits. Cell Rep. Phys. Sci. 101277 (2023).
Bai, S. et al. High-Efficiency Direct Methane Conversion to Oxygenates on a Cerium Dioxide Nanowires Supported Rhodium Single-Atom Catalyst. Nat. Commun. 11, 954 (2020).
Song, S. et al. A Selective Au-Zno/TiO2 Hybrid Photocatalyst for Oxidative Coupling of Methane to Ethane with Dioxygen. Nat. Catal. 4, 1032–1042 (2021).
He, Y. et al. In Situ Identification of Reaction Intermediates and Mechanistic Understandings of Methane Oxidation over Hematite: A Combined Experimental and Theoretical. Study. J. Am. Chem. Soc. 142, 17119–17130 (2020).
Wu, B. et al. Tandem Catalysis for Selective Oxidation of Methane to Oxygenates Using Oxygen over PdCu/Zeolite. Angew. Chem., Int. Ed. 61, e202204116 (2022).
Fanning, P. E. & Vannice, M. A. A DRIFTS Study of Cu–ZSM-5 Prior to and During Its Use for N2O Decomposition. J. Catal. 207, 166–182 (2002).
Zhao, W. et al. Fe-O Clusters Anchored on Nodes of Metal-Organic Frameworks for Direct Methane Oxidation. Angew. Chem., Int. Ed. 60, 5811–5815 (2021).
Wu, B. et al. Fe Binuclear Sites Convert Methane to Acetic Acid with Ultrahigh Selectivity. Chem 8, 1–15 (2022).
Sandoval, V. H. & Gigola, C. E. Characterization of Pd and Pd-Pb α-Al2O3 catalysts. A TPR-TPD study. Appl. Catal., A 148, 81–96 (1996).
Lee, J.-K. & Rhee, H.-K. Sulfur Tolerance of Zeolite Beta-Supported Pd−Pt Catalysts for the Isomerization of n-Hexane. J. Catal. 177, 208–216 (1998).
Henderson, M. A., Epling, W. S., Perkins, C. L., Peden, C. H. F. & Diebold, U. Interaction of Molecular Oxygen with the Vacuum-Annealed TiO2 (110) Surface: Molecular and Dissociative. Channels. J. Phys. Chem. B 103, 5328–5337 (1999).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (52164028, 22109035, 52274297, 22202053, 22378074, 52074099, 52164029) to X.L.T, P.L.D, J.L and S.K.Z. X.L.T, P.L.D, J.M.L, J.L, Z.Y.K and D.Q.W acknowledge the Start-up Research Foundation of Hainan University ((KYQD(ZR)-20008, 20082, 20083, 20084, 21124, 21125), the Central Government Guides Local Science and Technology Development Projects (ZY2022HN01), the collaborative Innovation Center of Marine Science and Technology, Hainan University (XTCX2022HYC04, XTCX2022HYC05), the specific research fund of The Innovation Platform for Academicians of Hainan Province (YSPTZX202315). Numerical computations were performed on Hefei advanced computing center. S.K.Z and J.M.L thanks the Natural Science Foundation of Hainan Province (221RC585), Hainan Province Science and Technology Special Fund (ZDYF2022GXJS004, ZDYF2023GXJS006).
Author information
Authors and Affiliations
Contributions
X.T. conceived and designed the experiments. Y.X. and Q.L undertook the materials synthesis, characterization, and performance testing. D.W. contributed to the DFT calculations. Q.Z. and L.G. performed the HAADF-STEM. P.R. assisted with the XANES. P.D. and L.G. co-supervised the experiments. M.T. assisted with the NMR. J.L, Y.H., C.W., S.Z., C.J., Z.L., Y.S., and Q.L assisted with data analysis and paper revision. Y.X., P.D., D.W., and X.T. co-wrote the paper. All the authors discussed the results and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Y., Wu, D., Zhang, Q. et al. Regulating Au coverage for the direct oxidation of methane to methanol. Nat Commun 15, 564 (2024). https://doi.org/10.1038/s41467-024-44839-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-024-44839-6
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.