Abstract
The active center for the adsorption and activation of carbon dioxide plays a vital role in the conversion and product selectivity of photocatalytic CO2 reduction. Here, we find multiple metal sulfides CuInSnS4 octahedral nanocrystal with exposed (1 1 1) plane for the selectively photocatalytic CO2 reduction to methane. Still, the product is switched to carbon monoxide on the corresponding individual metal sulfides In2S3, SnS2, and Cu2S. Unlike the common metal or defects as active sites, the non-metal sulfur atom in CuInSnS4 is revealed to be the adsorption center for responding to the selectivity of CH4 products. The carbon atom of CO2 adsorbed on the electron-poor sulfur atom of CuInSnS4 is favorable for stabilizing the intermediates and thus promotes the conversion of CO2 to CH4. Both the activity and selectivity of CH4 products over the pristine CuInSnS4 nanocrystal can be further improved by the modification of with various co-catalysts to enhance the separation of the photogenerated charge carrier. This work provides a non-metal active site to determine the conversion and selectivity of photocatalytic CO2 reduction.
Similar content being viewed by others
Introduction
The solar-energy-driven photocatalytic conversion of CO2 with H2O into hydrocarbon fuels is a significant solution for simultaneously addressing global energy demands and climate change issues1,2,3,4. Various products of CO2 reduction from photocatalytic multi-electron processes, including CO (two electrons), HCOOH (two electrons), HCHO (four electrons), CH3OH (six electrons), and CH4 (eight electrons), have been produced by a great variety of photocatalysts5,6,7,8. Achieving both high selectivity and high conversion for photocatalytic CO2 reduction is highly desirable in the field of photocatalysis research. However, efficient photoreduction of CO2 is very challenging, both in terms of chemical thermodynamics and kinetics, due to the highly stable structure of CO2 and the involvement of multiple proton-coupled electron transfer9,10,11. Additionally, the regulation of product selectivity in photocatalytic CO2 conversion remains an unknown challenge.
It has been well understood for the photocatalytic process that the identification of the active centers of catalysts for the adsorption and activation of CO2 is prerequisite for efficient CO2 conversion and product selectivity. Constructing an active center of catalysts for the adsorption and activation of CO2 is an efficient solution to improve CO2 conversion efficiency and product selectivity12,13,14. Various metal-free photocatalysts were reported for CO2 reduction15,16,17,18,19, typically such as covalent organic frameworks, graphitic carbon nitride, elemental phosphorus, boron nitride, and silicon carbide20,21,22,23,24. These metal-free photocatalysts have non-metallic sites as the adsorption and activation sites of CO2 molecules and thus photocatalytical CO2 reduction20,25,26,27,28. However, for metal oxide or sulfide photocatalysts, many studies suggest that metal components or defects on photocatalysts play a crucial role as primary sites in the adsorption and activation of CO2 and thus affect product selectivity29. Zhou et al. reported that the S vacancy or Cd vacancy CdS with single Au atom deposition for CO2 adsorption is different30. CO2 prefers to physically adsorb on single Au atoms of Au/CdS1−x and photoreduction into CO, while CO2 is more likely to chemically bond on the Cd vacancies of Au/Cd1−xS, resulting in a remarkable CO and CH4 generation rate on Au/Cd1−xS. He et al. synthesized a ZnIn2S4 nanosheet photocatalyst with abundant Zn vacancies31, where CO2 can be efficiently adsorbed on Zn vacancies to form CO2− species and highly selective photoreduction into CO. Yu et al. designed a Cu3SnS4 photocatalyst with S vacancies to increase ratios of Cu (I/II) for CO2 photoreduction32. The formed Cu (I) acts as adsorption sites for CO2, conducive to further hydrogenation of CO intermediate into CH4. Xie et al. showed that the defect-state CuIn5S8 ultrathin nanosheets have low-coordination Cu and In sites for CO2 adsorption to form highly stable Cu-C-O-In intermediates, which tend to obtain 100% CH4 selectivity33. Xu et al. designed a Co-Ni-P NH/BP catalyst with bimetallic sites to form a highly stable Co-O-C-Ni intermediate for the selective photoreduction of CO2 to CH434. However, the complex structures of defects on photocatalyst make it only a plausible correlation between defect structures and product selectivity. Some research shows that the adsorbed interaction between CO2 and metal sites is relatively weak since the formed metal-C bonds are weaker than the highly stable C=O bonds in CO2. This leads to the easy cleaving of metal-C bonds during the reaction process, hindering the deep reduction of CO2 into hydrocarbons30. Obviously, the non-metal sites on metal sulfide photocatalysts are very rarely considered the primary active center for the adsorption and activation of CO2.
Here, we have successfully prepared multiple metal sulfides, including CuInSnS4 octahedral nanocrystal and corresponding individual metal sulfides In2S3, SnS2, and Cu2S, through a simple one-step hydrothermal method. The CuInSnS4 nanocrystal is thermodynamically favorable to activate CO2 and leads to a switch of main products from CO to CH4 with a yield of 6.53 μL h−1 for the visible-light-driven CO2 reduction with H2O vapor without the assistance of any noble metal cocatalysts. In contrast, individual metal sulfides can only produce CO. We reveal that different adsorption configurations of CO2 on metal sulfides lead to different products in CO2 photoreduction. The non-metal sulfur atom in the prepared multiple metal sulfides CuInSnS4 octahedron nanocrystal is thermodynamically favorable to activate CO2 and leads to a switch of main products to CH4, as compared with the common individual metal sulfides In2S3, SnS2 and Cu2S with metal center as active sites to form CO products. CO2 is revealed to be adsorbed on the S atom center of CuInSnS4 to form an S-C-O-In structural unit, which is more conducive to protonation and leads to the efficient photocatalytic yield of CH4. Thus, we provide an insight into the role of non-metal center of photocatalyst in determining the conversion and selectivity of photocatalytic CO2 reduction. Although the pristine CuInSnS4 only exhibits a yield of CH4 evolution of 6.53 μL h−1 (corresponding to 5.83 μmol h−1 g−1), the activity and selectivity of CH4 evolution on CuInSnS4 can be significantly improved by modifying with cocatalysts such as Pt, CoO, NiO, and Co(OH)2. We believe that this knowledge can contribute to the development of more efficient and selective photocatalysts for CO2 reduction in the future.
Results
Characterization of CuInSnS4 octahedral nanocrystal
The as-prepared CuInSnS4 nanocrystals belong to the cubic spinel structure (JCPDS No. 29-0548), as revealed by the X-ray diffraction pattern (Fig. 1a), which were prepared through a simple one-step hydrothermal reaction. The hydrothermal temperature (160 °C, 180 °C, and 200 °C) did not have any evident impact on the crystalline and purity of CuInSnS4 (Supplementary Fig. 1). Under similar hydrothermal processes, In2S3 with a tetragonal crystal phase structure (JCPDS No. 25-0390), SnS2 with a hexagonal crystal phase (JCPDS No. 23-0677), and Cu2S with a cubic crystal phase were also prepared (JCPDS No. 02-1284) (Supplementary Fig. 2). The cubic spinel CuInSnS4 crystal structure shows that either In or Sn atom is coordinated with six S atoms to form an octahedron structure, while Cu atom is formed by a [CuS4] tetrahedral structural unit (Supplementary Fig. 3). For comparison, in In2S3 crystals, the In atom is present in [InS4] tetrahedron and [InS6] octahedron coordination (Supplementary Fig. 4). Cu and Sn atoms exist as [CuS4] tetrahedron and [SnS6] octahedron coordination in Cu2S and SnS2 crystal structure, respectively (Supplementary Figs. 5, 6). The coordination structure of each metal in CuInSnS4 coincides with the individual Cu2S, In2S3, and SnS2. The composition of CuInSnS4 is analyzed by energy-dispersive spectroscopy, as shown in Fig. 1b. EDS offers that the Cu, Sn, In, and S atomic ratio is ~1:1:1:4, very close to the stoichiometric value of CuInSnS4 compounds, indicating the high purity of CuInSnS4 nanocrystal. Meantime, the ICP-MS test results of the metal element content of the nano-single crystal CuInSnS4 sample are presented in Table S1. The atomic ratio of Cu:In:Sn is 1.06:1.00:1.00, which closely matches the theoretical value of CuInSnS4. Supplementary Fig. 7 shows the SEM images of In2S3, Cu2S, and SnS2 samples. In2S3 exhibits a morphology of microspheres self-assembled from nanosheets. Cu2S has the basic shape of nanoparticles, while SnS2 displays the morphology of ultrathin nanosheets. Both scanning electron microscopy and transmission electron microscopy images of nanoscale microstructure confirm that the prepared CuInSnS4 displays an octahedral nanocrystal structure with a size of about 30 nm (Supplementary Fig. 8 and Fig. 1c). The high-resolution TEM image (Fig. 1d) shows the exposed facets of the octahedron with a lattice spacing of 0.601 nm, which is assigned to (1 1 1) facets of CuInSnS4. Selected area electron diffraction further verifies that the CuInSnS4 sample not only exposes the (1 1 1) crystal plane but also has a single crystal structure (Fig. 1e). All the results confirm the successful preparation of CuInSnS4 nanocrystal with high-quality exposed (1 1 1) crystal faces. The specific surface area of the CuInSnS4 sample is about 24.1 m2 g−1, while that of the prepared In2S3, SnS2, and Cu2S are 26.8 m2 g−1, 37.6 m2 g−1, and 3.6 m2 g−1, respectively (Supplementary Fig. 9). As displayed in Fig. 1f–i, the uniform distribution of Sn, S, In, and Cu elements in CuInSnS4 octahedral nanoparticles indicates that the catalyst is of high purity.
X-ray photoelectron spectroscopy was used to compare the electronic states of the obtained sample. The Cu2p3/2 and Cu2p1/2 binding energies of CuInSnS4 sample are 932.07 eV and 951.90 eV, respectively (Fig. 2a). This demonstrates that the valence state of Cu is +1 in the CuInSnS4 sample35,36, which is also confirmed by the Cu LMM spectra (Supplementary Fig. 10). Notably, the Cu2p-binding energies of the CuInSnS4 sample is identical to that of Cu2S. The binding energies of In3d5/2 and In3d3/2 in the CuInSnS4 sample are 444.63 eV and 452.18 eV, respectively. These values indicate that the valence state of In in the CuInSnS4 sample is +3. Compared with In2S3, the In3d binding energy of CuInSnS4 uniformly shifts toward the lower binding energy, as shown in Fig. 2b. This is attributed to the difference in the In coordinated environment between CuInSnS4 and In2S3 because the partial In atom in In2S3 exists in the state of [InS4] tetrahedron37. In the CuInSnS4 sample, the Sn3d5/2 and Sn3d3/2 doublets are centered respectively at 486.30 eV and 494.70 eV, assigning to Sn4+ valence state. Notably, the binding energy of Sn in CuInSnS4 is slightly lower than that in the parent SnS2 (Fig. 2c). The possible reason is that the Sn atoms are in different crystal structures38. Furthermore, the binding energies of S2p3/2 and S2p1/2 in the CuInSnS4 sample are measured to be 161.45 eV and 162.70 eV, respectively, which corresponds to the S2− valence state. In the S2p XPS spectra, the binding energies of S atoms increase in the order of Cu2S < In2S3 < SnS2 < CuInSnS4, as shown in Fig. 2d. S atoms in CuInSnS4 have the highest binding energy. This is interpreted by the fact that the average bond length (0.253 nm) between sulfur and metal atoms in CuInSnS4 is slightly larger than in monometallic sulfide4,37,39. Moreover, the S atom in CuInSnS4 has a higher binding energy than that of monometallic sulfides, indicating an electron-deficient state of the S atoms in CuInSnS4 compared to monometallic sulfides. This electron-deficient state of the S atom in CuInSnS4 can serve as the reaction site for CO2 adsorption and activation.
Photocatalytic conversion of CO2 and H2O vapor
The photocatalytic CO2 reduction performance of the samples was evaluated in a customized sealed quartz glass vessel, in a gas-solid reaction system, with a small amount of water vapor in a CO2 atmosphere, under the irradiation of a 300 W Xe lamp with a 420 nm cutoff wavelength filter (Supplementary Fig. 11). It is important to note that this reaction is a gas-solid phase reaction. The system contains only 50 μL of water, which evaporates into water vapor upon injection into the reactor. As a result, only gaseous products such as CH4, CO, and a small amount of H2 are detected, while liquid products (such as CH3OH, HCHO, HCOOH, etc.) are not detected. Figure 3a shows the corresponding photocatalytic CO2 performance of the samples under visible-light irradiation. For single metal sulfides In2S3, Cu2S, and SnS2, CO is the main product with a yield rate of less than 3.2 μL h−1 from photocatalytic CO2 reduction, while the multi-electron transfer product CH4 is hardly formed. Whereas, the CuInSnS4 sample shows excellent photocatalytic CO2 reduction performance, yielding CH4 as the main product besides a slight amount of CO and H2 evolution. The hydrothermal temperature has no obvious impact on the CO2 reduction performance of CuInSnS4 (Supplementary Fig. 12). The rate of CH4 generation reaches 6.53 μL h−1 for the CuInSnS4 sample. The selectivity of CH4 is calculated to be 67.3% based on the contents of carbon-containing products. The significant difference in the product selectivity demonstrates the different mechanisms of CO2 reduction or the different active sites between CuInSnS4 and single metal sulfides. The controlled blank experiments under other conditions were investigated to confirm the occurrence of CO2 reduction on CuInSnS4, as shown in Supplementary Fig. 13. The CH4 product is not detected without either light irradiation or catalyst, proving that CO2 reduction is a light-induced catalytic reaction on CuInSnS4. Meanwhile, without adding H2O into the reaction system, only a very few products are detected, indicating that H2O is also one of the essential reactants involved in the reaction. When N2 is used instead of CO2 for the reaction, only a small amount of CO is detected, directly proving that the source of CO and CH4 products is CO2. The presence of small amounts of CO may be attributed to contamination from ambient air, reactors, and equipment, as we can see that small CO products is also detected without CuInSnS4 photocatalysts40. Figure 3b shows the stability of the CuInSnS4 sample for photocatalytic reduction of CO2. It is clear that the CuInSnS4 sample presents good performance without noticeable activity decrement after three-cycle photocatalytic CO2 reduction tests of a total of 27 h (9 h visible-light irradiation for each cycle). Neither crystal structural transformation nor absorption behavior changes are found in the XRD pattern and ultraviolet–visible diffuse reflectance spectra for the CuInSnS4 sample after photocatalytic reaction (Supplementary Fig. 14). These results suggest that CuInSnS4 possesses good stability during photocatalytic CO2 reduction. Moreover, the XPS of the catalyst after the reaction shows that a weak photocorrosion phenomenon occurs in CuInSnS441,42 (Supplementary Fig. 15). Specifically, the photogenerated holes or the active oxygen species oxidize the surface S2− of the catalyst to SO32−. The peaks with binding energies in the range of 168.26~170.26 eV are assigned to the XPS peaks of SO32− species (Supplementary Fig. 16). The photocatalytic performance of CuInSnS4 sample is evaluated under different monochromatic light wavelengths in Fig. 3c. It is observed that as the wavelength of the incident light increases, the photocatalytic activity significantly decreases. However, the CuInSnS4 nano-single crystal photocatalyst demonstrates a CH4 generation rate of 0.69 μL h−1 and CO generation rate of 0.22 μL h−1 under the irradiation of 525 nm monochromatic light, which is surprising. The different monochromatic light tests indicate that CuInSnS4 is an exceptional catalyst for CO2 photoreduction under visible light. The 13CO2 isotope experiment further validates that CH4 product is generated from the photoreduction of CO2 molecules, where only 13CH4 is detected when the reaction is carried out in a 13CO2 atmosphere, as shown in Fig. 3d. Meanwhile, the 13CO2 isotope also confirms that the generated CO was indeed a product of CO2 photoreduction. When the reaction is conducted in a 13CO2 atmosphere, the weak peak of 13CO with a m/z = 29 was detected due to the low activity of CuInSnS4 for CO evolution (Supplementary Fig. 17a). It is noteworthy that the mass spectrum peak at m/z = 28 corresponds to the N2 molecule from air interference, as evident from the distinct N2 peaks in chromatogram. Additionally, the 12CO2 experiment further evidences that the generated CO results from the photoreduction of CO2 molecules with no peaks at m/z = 29 (Supplementary Fig. 17b).
Although the pristine CuInSnS4 only exhibits a yield of CH4 evolution of 6.53 μL h−1 (corresponding to 5.83 μmol h−1 g−1) with a selectivity of 67.3%, the activity and selectivity of CH4 evolution on CuInSnS4 can be improved by coupling with semiconductor photocatalysts or noble metals as cocatalysts. We evaluated the photocatalytic performance of CuInSnS4 modified with Pt, CoO, NiO, Co(OH)2, and dual co-catalysts Pt and Co(OH)2, for the CO2 reduction reaction. The composition and chemical states of Pt, CoO, NiO, Co(OH)2 cocatalysts are well verified by XRD patterns and XPS spectra (Supplementary Figs. 18–23). Table S2 lists a comparison of the photoreduction activity of CuInSnS4 and co-catalyst-modified CuInSnS4 photocatalysts, along with the common metal sulfide systems currently used for CO2 photoreduction. Clearly, both the yield and selectivity of CH4 evolution on CuInSnS4 can be significantly improved by modifying with cocatalysts such as Pt, CoO, NiO, and Co(OH)2. The activity of CH4 evolution on the modified CuInSnS4 photocatalysts surpasses the majority of the reported photocatalysts for CO2 reduction up to now. Particularly, the incorporation of Co(OH)2 as a co-catalyst significantly enhances the CO2 photoreduction activity of the CuInSnS4 photocatalyst. As the Co(OH)2 loading increases, the photoreduction activity of CO2 exhibits a characteristic volcanic pattern. With 5%Co(OH)2 loading onto CuInSnS4, the production rates for CH4 and CO, respectively, reach 145.45 and 32.32 μmol h−1 g−1, corresponding to a CH4 selectivity of 81.8%. The generation rates of CH4 and CO are 25 times and 13 times that of pure CuInSnS4, respectively. Furthermore, when CuInSnS4 is modified with a dual co-catalyst of 5% Co(OH)2 as an oxidation co-catalyst and 1%Pt as a reduction co-catalyst, CH4 production reaches 195.60 μmol h−1 g−1, along with 22.00 μmol h−1 g−1 of CO, and a CH4 selectivity of 89.9%. Photoelectrochemical characterization was employed to assess the separation efficiency of photogenerated carriers on the modified CuInSnS4, as shown in Supplementary Fig. 24. Clearly, the CuInSnS4 samples modified with the co-catalyst exhibit a higher photocurrent signal and a smaller electrochemical impedance radius as compared to the parent CuInSnS4 sample. The photocurrent increases sequentially in the order of CuInSnS4 < 5%Co(OH)2/CuInSnS4 ≈ 1%Pt/CuInSnS4 < 5%Co(OH)2/CuInSnS4/1%Pt, indicating that the modification of the dual co-catalyst improves photoelectric carrier separation and migration compared to the single co-catalyst. The NiO and CoO cocatalysts also improve the separation efficiency and migration rate of the photogenerated carriers of CuInSnS4 photocatalyst. The decreasing order of electrochemical resistance radius is CuInSnS4 > 1%Pt/CuInSnS4 ≈ 5%Co(OH)2/CuInSnS4 > 5%Co(OH)2/CuInSnS4/1%Pt, consistent with the photocatalytic activity trend. Additionally, both 10%NiO/CuInSnS4 and 10%CoO/CuInSnS4 exhibit smaller electrochemical resistance radii than pure CuInSnS4, confirming that the cocatalyst promotes the photogenerated charge separation and migration. Therefore, the modification of CuInSnS4 with various cocatalysts to enhance the separation of photogenerated carriers is related to the activity and selectivity of CH4 products. The apparent quantum yield is calculated by measuring the yield of CH4 and CO in 5%Co(OH)2/CuInSnS4/1%Pt under monochromatic light at 400 nm. Supplementary Fig. 25 shows the spectrum and intensity of monochromatic light at 400 nm. Under 400 nm monochromatic light irradiation, the apparent quantum efficiencies for CH4 and CO are 0.16% and 0.01%, respectively. Based on the above analysis, we believe that CuInSnS4 nano-single crystal photocatalysts through further optimization design of the different contents and types of cocatalyst modification can be more efficient and selective for CO2 reduction in the future.
Energy band and photoelectrochemical characterization
The band energy potential is a key determinant of the driving force of redox reactions. Therefore, we have studied the band structure of the catalyst through UV-vis DRS and XPS valence band spectroscopy. As shown in Fig. 4a and Supplementary Fig. 26, the optical absorption band edge of CuInSnS4 is calculated to be 787.5 nm, which corresponds to a band gap energy of 1.57 eV. For comparison, the absorption band edges of single metal sulfide Cu2S, SnS2, and In2S3 are 747.0 nm, 552.7 nm, and 641.6 nm, corresponding to the band gap of 1.66 eV, 2.24 eV, and 1.93 eV, respectively. Moreover, the valence band potential of CuInSnS4 is determined to be 0.50 V from the valence band XPS spectra (Supplementary Fig. 27), while the Cu2S, SnS2, and In2S3 possess valence band potentials of 1.02 V, 1.70 V, and 1.93 V, respectively. By using the formula ECB = Eg–EVB, we have determined that the conduction band potentials of CuInSnS4, Cu2S, SnS2, and In2S3 are −1.15 V, −1.10 V, −0.54 V, and −0.55 V, respectively. Based on the optical band gaps, we have obtained the electronic band energies relative to a normal hydrogen electrode (Fig. 4b), indicating that both CuInSnS4 and single metal sulfides have the ability to reduce CO2 to CH4 and CO. Notably, CuInSnS4 exhibits the highest reduction potential for the photogenerated electrons to reduce CO2. Additionally, CuInSnS4 shows a significantly increased photocurrent density (0.014 mA cm−2) compared to the single metal sulfide under visible-light irradiation (Fig. 4c), indicating a more efficient separation of the photoinduced charge in multi-metal sulfides. The lower interface resistance in the corresponding electrochemical impedance spectra (Fig. 4d) confirms the rapid transfer of photogenerated electrons in CuInSnS4. The efficient separation efficiency and migration rate of photogenerated carriers make polymetallic sulfides exhibit higher photocatalytic CO2 reduction performance compared to monometallic sulfides. However, the higher migration and separation efficiency of charge carriers in CuInSnS4 alone is not sufficient to explain the substantial difference in the product selectivity between CuInSnS4 and single metal sulfides.
The in situ CO2 adsorption FT-IR spectra and mechanism
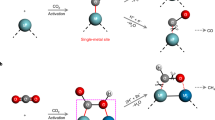
To understand the CO2 reduction process over CuInSnS4 and single metal sulfides, in situ Fourier-transform infrared spectroscopy is used to compare the reaction intermediates on the catalyst surface. No macroscopic infrared absorption peaks for intermediates are found on Cu2S or SnS2, even under light irradiation, possibly due to their weak chemical interaction with CO2 (Supplementary Fig. 28a, b). However, In2S3 shows a significant activation effect on the CO2 adsorbed on the surface under light irradiation (Fig. 5a). Notably, CO2 can form chemical adsorption with In2S3 even under dark conditions, as indicated by the infrared peak at 1150 cm−1, which can be considered an O-S stretching vibration43, suggesting that the oxygen atom of CO2 is bonded to the sulfur atom of In2S3. Upon light irradiation, some infrared peaks of the produced intermediates on the catalyst surface are observed. The infrared peak at 1225 cm−1 is attributed to the vibration of bidentate bicarbonate44, while the infrared peak at 1412 cm−1 is attributed to the vibration of monodentate bicarbonate45. Most importantly, the infrared peak at 1610 cm−1 is attributed to the *COOH group, which is generally regarded as the crucial intermediate for CO2 reduction to CO46. Surprisingly, the polymetallic sulfide CuInSnS4 exhibits strong chemisorption of CO2 and strong physisorption of H2O (Fig. 5b). The prominent infrared peak observed at 1627 cm−1 is attributed to the physical adsorption of H2O. However, In2S3 does not exhibit a noticeable infrared adsorption peak of H2O at 1627 cm−1. This is because In2S3 has a lower affinity towards water adsorption than CuInSnS4, as indicated by the high contact angle on In2S3 surface (Supplementary Fig. 29). Moreover, a prominent infrared peak at 1117 cm−1 assigned to C-S stretching vibration is observed upon the CO2 adsorption on CuInSnS447,48,49. The CO2 adsorption and activation are significantly improved on CuInSnS4 compared to In2S3. Furthermore, the adsorption state of CO2 on CuInSnS4 is different from that on In2S3, as the carbon atom of CO2 is bonded to the sulfur atom of CuInSnS4. Under light conditions, CuInSnS4 produces specific CO2-activated intermediates, as indicated by the infrared peaks at 1225 cm−1 and 1260 cm−1, attributed to the vibration of bidentate bicarbonate and bidentate carbonate50,51, respectively. Additionally, the infrared peaks at 1100 cm−1 and 1160 cm−1 are attributed to the absorption peaks of *CHO and *CH3O, which are intermediates for the yield of CH452,53. Therefore, either the CO2 adsorption state in darkness or the produced intermediates under light irradiation show that the sites for CO2 adsorption and the CO2 reduction approach differ between single-metal sulfide In2S3 and multi-metal sulfide CuInSnS4. This may account for the different selectivity of products between In2S3 and CuInSnS4.
It has been demonstrated that sulfur defect sites in multiple metal sulfides acted as an active center for the selective photoreduction of CO2 to CH4. However, CuInSnS4 nano-single crystal shows no significant sulfur defect signals compared to the strong defect signals in SnS2 and In2S3, as shown in Supplementary Fig. 30. This indicates that the selectivity of CH4 products on CuInSnS4 is not related to sulfur defects. The mechanism of selective photocatalytic CO2 reduction on CuInSnS4 and In2S3 photocatalysts is further theoretically studied. Firstly, we investigated the adsorption behavior of CO2 on the surfaces of In2S3 and CuInSnS4. The (0 0 1) crystal plane of the In2S3 sample and the (1 1 1) crystal plane of the CuInSnS4 sample were selected as models, and all atoms on the crystal planes were considered as potential sites for CO2 adsorption activation (Supplementary Figs. 31 and 32). Based on CO2 adsorption energy, the optimal adsorption models of CO2 on In2S3 and CuInSnS4 photocatalyst surfaces are optimized (Supplementary Figs. 33 and 34). Figure 5c, d depict schematic diagrams of CO2 stable adsorption configurations and the charge density difference of CO2 on In2S3 and CuInSnS4, respectively. The stable CO2 adsorption configuration on In2S3 is the C atom of CO2 bonded to In atom with a bond length of 2.27 Å, while the O atom of the CO2 molecule is bonded with the S atom with a bond length of 1.70 Å. The CO2 adsorption model for the polymetallic sulfide CuInSnS4 is the opposite. The unique C-S bond with a bond length of 1.90 Å is formed between the C atom of CO2 and the surface S atom, and one O atom of CO2 is bonded with In atom with a bond length of 2.23 Å. The various adsorption configurations of CO2 on In2S3 and CuInSnS4 surfaces are attributed to the coordination environment and charge number of the surface S atoms. The surface S atom of In2S3 is an electron-rich site with [SIn3] coordination structure, while the S atom on CuInSnS4 is an electron-poor center with [SInSnCu] coordination structure (Supplementary Fig. 35 and Table S1). Different adsorption configurations may be the key to determining the direction of electron transfer and thus the selectivity of CO2 reduction on CuInSnS4 and In2S3. Adsorption of CO2 on the In2S3 (0 0 1) crystal plane leads to inconsistent changes in two C=O lengths. The length of the C-O2 bond is 1.43 Å, equal to the ordinary C-O (1.43 Å) single bond, while the length of C-O1 is shortened to 1.19 Å, close to the length of C-O (1.12 Å) in a CO molecule. This asymmetric activation is more likely to cause the rupture of C-O2, thereby preferentially producing CO on In2S3. In the case of the CuInSnS4 (1 1 1) crystal plane, both C-O bonds are similar in length, measuring 1.26 ± 0.02 Å. They are longer than the C-O bond (1.16 Å) in a free CO2 molecule, indicating that the bond energy of two C=O bonds of the activated CO2 is simultaneously weakened. The calculation of the charge density difference reveals the difference in electronic structure and electron flow resulting from the interaction of CO2 with the surface atoms of In2S3 and CuInSnS4. On the In2S3 surface, there is extensive charge depletion for the C-O2 and S–O2 bonds, which implies that these chemical bonds are weakened. In contrast, CO2 exhibits a wide charge accumulation region on CuInSnS4, leading to the formation of a strong C-S and In-O chemical bond. This strong interaction is beneficial for the firm adsorption of CO2 on the CuInSnS4 surface, promoting the further deep reduction reaction. The Bader charges analysis further confirms that there is more charge transfer between the CuInSnS4 surface and CO2 molecules adsorbed on it. The surface of In2S3 and CuInSnS4 loses 0.28e and 0.32e, respectively, after CO2 adsorption (Table S3).
The stable configuration of CO2 adsorption on CuInSnS4 determines its excellent CO2 photoreduction activity and selectivity. Therefore, DFT calculations were further carried out to study the conversion pathway of CO2 on the CuInSnS4 photocatalyst surface, as shown in Fig. 6. In Fig. 6a, the adsorption configuration of CuInSnS4 is shown for each intermediate step, from CO2 adsorption to CH4 generation. The C atoms of various intermediates, such as CO2*, COOH*, CHO*, CH2O*, and CH3*, remain in a stable bond to the electron-deficient S atoms of the CuInSnS4 nano-single crystal (1 1 1) plane. With the addition of protons and electrons, the removal of the H2O molecule results in the breaking of the chemical bond between O atoms of intermediates and In atoms on CuInSnS4. Moreover, unlike most metal sulfide photocatalysts, the hydrogenation of CO2 adsorbed on the CuInSnS4 surface to COOH* is an easy step, requiring only a potential energy barrier of 0.075 eV33, as shown in Fig. 6b. This is attributed to the fact that the electrons are localized on the O2 atom of CO2 molecules in the S-C-O-In adsorption configuration of CO2 on the CuInSnS4 surface to facilitate the addition of protons, thus lowering the formation energy of COOH* intermediates. However, converting COOH* to CO is an endothermic reaction that must overcome an energy barrier of 0.46 eV. On the other hand, the continuous hydrogenation of COOH* intermediates to produce HCOOH* is an exothermic reaction, promoting the hydrogenation of CO2. The formation of H2CO* intermediates is the rate-limiting step for further hydrogenation processes, but the hydrogenation of H2CO* to H3CO* and finally to CH4 is thermodynamically spontaneous. Therefore, CuInSnS4 can achieve high selectivity for CH4 products. Additionally, the adsorption energies of each intermediate product can explain the high CH4 selectivity. Supplementary Fig. 36 shows that the adsorption energies of CH4, CH3OH, and HCOOH on the CuInSnS4 surface are −0.17, −0.61, and −0.67 eV, respectively, with CH4 having the highest adsorption energy. This indicates that CH4 products are most easily desorbed from the CuInSnS4 surface, which is one of the reasons why the CuInSnS4 photocatalyst has high selectivity for the photoreduction of CO2 into CH4.
The photoreduction mechanism of CO2 on the CuSnInS4 surface is proposed in Fig. 7. The first step involves CO2 adsorbing on the catalyst surface to form the unique S-C-O-In structural unit. This process weakens the C=O double bond in the CO2 molecule while non-metallic S atoms serve as adsorption sites, ensuring a strong bond to the C atom of CO2. This benefits the continuous reduction of CO2 molecules into COOH*, HCO*, H3CO* intermediates, and ultimately into CH4 on the catalyst surface through the assistance of photogenerated electrons and protons. Lastly, the low adsorption energy of CH4 on the catalyst surface facilitates its quick release, completing the full photocatalytic cycle reaction.
Proposed photocatalytic mechanism for CO2 reduction on the CuInSnS4. The backgroup crystal structure was created by VESTA program56.
In summary, a CuSnInS4 nano-single-crystal photocatalyst with exposed (1 1 1) facets is successfully prepared by a simple one-step hydrothermal reaction. Under visible-light irradiation, the CuSnInS4 nano-single crystal photocatalyzes the conversion of CO2 and H2O into main products of CH4 with a generation rate of 6.53 μL h−1, significantly higher than that of single metal sulfides (In2S3, Cu2S, and SnS2). The electron-poor center sulfur atom on the CuSnInS4 (1 1 1) crystal plane acts as the site for CO2 adsorption and activation, which leads to the activation of the two symmetrical C=O double bonds of CO2 molecule to form a stable S-C-O-In transition state. This induces CH4 generation via the conversion route of COOH*→HCOOH*→H2CO*→H3CO*→CH4*. However, the asymmetric activation of CO2 by monometallic sulfides is more likely to result in the cleavage of individual C-O bonds in the CO2 molecule, leading to the preferential photoreduction of CO2 to CO. This work provides a distinctive understanding of catalysts for CO2 adsorption and activation for the CO2 selective conversion to help the conversion of CO2 resources into high-value-added products.
Methods
Preparation of CuInSnS4 nanocrystal
A simple one-step hydrothermal method was used to synthesize CuInSnS4 nanocrystal photocatalyst with the cubic crystal structure. The detailed operation process is as follows. Firstly, 1 mmol of CuCl, 1 mmol of SnCI4·5H2O, and 1 mmol of InCI3·4H2O were added to 40 mL of deionized water to form a solution under vigorous stirring. Then, 5 mmol of TAA was dissolved in the above-mixed solution and stirred at room temperature for 30 min. Finally, the resulting mixed solution was transferred to a 50 mL Teflon-lined autoclave and sealed into a stainless steel tank for hydrothermal reaction. The hydrothermal temperature is controlled at 160, 180, and 200 °C for 24 hours. After the reaction, the product was collected and washed with deionized water, and dried under vacuum at 60 °C. The obtained samples were labeled CuInSnS4 (160 °C), CuInSnS4 (180 °C), and CuInSnS4 (200 °C) according to the reaction temperature. The detailed preparation processes of CuInSnS4 modified Pt, CoO, NiO, and Co(OH)2 cocatalysts can be seen in supplementary information.
Characterization
X-ray diffractometer (D8 Advance, Bruker) was used to analyze the crystal structure of the catalyst. The XRD test range is 10°−80°, and the scan rate is 10° min−1. Scanning electron microscopy (su8010, Hitachi) was used to observe the surface morphology of the catalyst. The element composition and ratio of the sample are detected by EDS. The apparent morphology and high-resolution TEM image of the catalyst were tested by transmission electron microscope (TEM, TECNAI G2F20, FEI Company). At the same time, SAED and element mapping images of the catalyst were obtained in the TEM measurement mode. A UV-VIS-NIR Spectrophotometer (Cary 500) was used to obtain the catalyst UV-VIS-NIR DRS, in which BaSO4 was used as a standard sample for 100% light transmission. The Micromeritics 3020 M physical adsorption instrument was used to obtain the nitrogen adsorption and desorption curves of different catalysts. The specific surface area of each catalyst was calculated from the type of nitrogen adsorption and desorption curves. The catalyst and dried potassium bromide were evenly ground, and 20 mg was weighed and pressed into slices, then placed in a quartz infrared tube for a carbon dioxide adsorption infrared test. In situ infrared spectra measurements were performed using a Fourier-transform infrared spectrometer (Nicolet iS50 FT-IR Spectrometer) equipped with a mercury cadmium telluride detector (Supplementary Fig. 37). In situ infrared spectra were recorded by averaging 32 scans at a resolution of 4 cm−1. To initiate the experiment, the catalyst was placed in a 250 mL quartz tube and compacted into a film. The tube was then subjected to vacuum treatment for 60 min. Subsequently, high-purity CO2 gas was introduced, and the quartz tube was sealed. A liquid sampler was used to inject 60 μL of deionized water into the sealed quartz tube. The tube was heated with a hot blower to vaporize the deionized water. The quartz tube was positioned in the FT-IR spectrometer, ensuring that the incident light of the infrared spectrometer was perpendicular to the sample surface. A xenon lamp visible-light source was introduced to directly illuminate the sample surface. Infrared spectra were recorded after pretreating the catalyst in a vacuum, introducing CO2 gas, and vaporizing deionized water, respectively. After the introduction of light, infrared spectra were recorded every 5 min. Gas chromatography-mass spectrometry (Agilent 7890B, Agilent 5977B MSD) was used to analyze 13CH4 and 13CO. Electron paramagnetic resonance spectroscopic measurements were performed at room temperature using a Bruker A300 EPR spectrometer.
Photocatalytic performance
300 W xenon lamp (Microsolar 300, Beijing Perfectlight Technology Co., Ltd.) was equipped with a 420 nm cutoff wavelength filter as a light source that simulates visible light for photocatalytic CO2 reduction tests. Firstly, 50 mg of catalyst was dispersed in 5 mL of deionized water and sonicated for 10 min. Then the catalyst was dropped into a watch glass with a diameter of 3 cm and dried at 80 °C. Subsequently, the dried catalyst was placed in a quartz reactor with a volume of ~250 cm3, and then high-purity CO2 gas (99.999%) was introduced to replace all the air. The flow rate of carbon dioxide gas was ~100 mL/min and lasted for 1 h. Finally, 50 μL deionized water was injected into the quartz reactor from the rubber stopper through a gas chromatography liquid syringe (maximum range, 50 μL), and the reactor was heated with a hair dryer to evaporate the water into water vapor. As a result, water is present in the form of water vapor throughout the reaction. The reactor was placed under a xenon lamp for photocatalytic reaction, and the current of the xenon lamp was 16 A. After 1 h of illumination, 0.5 mL of gas was extracted from the reactor with a 1 mL gas chromatograph gas syringe and injected into the gas chromatograph for product analysis and detection. Among them, H2, O2, and N2 are detected by a thermal conductivity detector. CH4 was detected by the flame ionization detector. CO passes through the flame ionization detector after being transformed by the nickel reformer. The product was qualitatively and quantitatively analyzed by gas chromatography retention time and appearance standard curve method.
Theoretical calculations
The density functional theory calculations were performed using the VASP code with the projected augmented wave method54. Generalized gradient approximation in the scheme of Perdew-Bueke-Ernzerhof was used for the exchange-correlation functional54. The PBEsol exchange-correlation function was adopted in the optimization calculations. Grimme’s DFT-D3 scheme was used to describe the long-range vdW interactions54. The cutoff energy of the basis function was 420 eV. For the CuSnInS4 (1 1 1) crystal plane, a 2 × 2 × 1 supercell with a four-layer slab was constructed, and only the top two layers were allowed to relax. A vacuum region of 12 Å was set above the surface for periodic boundary conditions, and dipole correction was also applied. Gamma-centered 1 × 1 × 1 grid k-points were used for the interface. Geometry relaxation was performed until the energy, and atomic forces converged to be smaller than 10−5 eV and 0.03 eV/Å. Charge transfers were calculated using the Bader charge analysis54,55.
The free energy of each reaction intermediate was determined by: G = E + ZPE–TS. The electronic energy was directly obtained from DFT calculations. The zero-point energy and entropy correction (TS, T = 298.15 K) were computed from vibration analysis according to standard methods. The adsorption-free energy of the adsorbates can be calculated using: ∆Gads = ∆Eads + ∆ZPE–T∆S, where ∆Eads is the adsorption energy of the adsorbates, and ∆ZPE and ∆S are the difference between ZPE and S, respectively. After the adsorption-free energies of the adsorbates are obtained, the reaction-free energies of CO2 reduction reaction elementary steps can be determined correspondingly by using the computational hydrogen electrode model40.
Data availability
The authors declare that the data supporting the findings of this study are available in the paper and its supplementary information files. Source data are provided with this paper.
References
Zhang, Q. et al. Designing covalent organic frameworks with Co-O4 atomic sites for efficient CO2 photoreduction. Nat. Commun. 14, 1–11 (2023).
Xu, R. et al. Tandem photocatalysis of CO2 to C2H4 via a synergistic rhenium-(I) bipyridine/copper-porphyrinic triazine framework. J. Am. Chem. Soc. 145, 8261–8270 (2023).
Shen, Y. et al. Room-temperature photosynthesis of propane from CO2 with Cu single atoms on vacancy-rich TiO2. Nat. Commun. 14, 1–9 (2023).
Chai, Y. et al. Distortion of the coordination structure and high symmetry of the crystal structure in In4SnS8 microflowers for enhancing visible-light photocatalytic CO2 reduction. ACS Catal. 11, 11029–11039 (2021).
Shi, X. et al. Highly selective photocatalytic CO2 methanation with water vapor on single-atom platinum-decorated defective carbon nitride. Angew. Chem. Int. Ed. 61, e2022030 (2022).
Park, H. R., Pawar, A. U., Pal, U., Zhang, T. & Kang, Y. S. Enhanced solar photoreduction of CO2 to liquid fuel over rGO grafted NiO-CeO2 heterostructure nanocomposite. Nano Energy 79, 105483 (2021).
Kamada, K. et al. Photocatalytic CO2 reduction using a robust multifunctional iridium complex toward the selective formation of formic acid. J. Am. Chem. Soc. 142, 10261–10266 (2020).
Ding, J. et al. Core–shell covalently linked graphitic carbon nitride–melamine–resorcinol–formaldehyde microsphere polymers for efficient photocatalytic CO2 reduction to methanol. J. Am. Chem. Soc. 144, 9576–9585 (2022).
Hao, Y. C. et al. Metal-organic framework membranes with single-atomic centers for photocatalytic CO2 and O2 reduction. Nat. Commun. 12, 1–11 (2021).
Xia, Y. et al. Highly selective CO2 capture and its direct photochemical conversion on ordered 2D/1D heterojunctions. Joule 3, 2792–2805 (2019).
Zhu, J. et al. Asymmetric triple-atom sites confined in ternary oxideenabling selective CO2 photothermal reduction to acetate. J. Am. Chem. Soc. 143, 18233–18241 (2021).
Liu, Y. et al. Improving CO2 photoconversion with ionic liquid and Co single atoms. Nat. Commun. 14, 1–11 (2023).
Tan, T. H. et al. Unlocking the potential of the formate pathway in the photo-assisted Sabatier reaction. Nat. Catal. 3, 1034–1043 (2022).
Yang, S. et al. Microenvironments enabled by covalent organic framework linkages for modulating active metal species in photocatalytic CO2 Reduction. Adv. Funct. Mater. 32, 2110694 (2022).
Nguyen, H. L. & Alzamly, A. Covalent organic frameworks as emerging platforms for CO2 photoreduction. ACS Catal. 11, 9809–9824 (2021).
Guo, R. T., Zhang, Z. R., Xia, C., Li, C. F. & Pan, W. G. Recent progress of cocatalysts loaded on carbon nitride for selective photoreduction of CO2 to CH4. Nanoscale 15, 8548–8577 (2023).
Zhai, R. et al. A review of phosphorus structures as CO2 reduction photocatalysts. Small 19, 2207840 (2023).
Laghaei, M., Ghasemian, M., Lei, W., Kong, L. & Chao, Q. A review of boron nitride-based photocatalysts for carbon dioxide reduction. J. Mater. Chem. A 11, 11925–11963 (2023).
Li, C., Xu, Y., Tu, W., Chen, G. & Xu, R. Metal-free photocatalysts for various applications in energy conversion and environmental purification. Green. Chem. 19, 882–899 (2017).
Biswas, S., Dey, A., Rahimi, F. A., Barman, S. & Maji, T. K. Metal-free highly stable and crystalline covalent organic nanosheet for visible-light-driven selective solar fuel production in aqueous medium. ACS Catal. 13, 5926–5937 (2023).
Yuan, Y. P., Cao, S. W., Liao, Y. S., Yin, L. S. & Xue, C. Red phosphor/g-C3N4 heterojunction with enhanced photocatalytic activities for solar fuels production. Appl. Catal., B 140-141, 164–168 (2013).
Hu, Z., Lu, Y., Liu, M., Zhang, X. & Cai, J. Crystalline red phosphorus for selective photocatalytic reduction of CO2 into CO. J. Mater. Chem. A 9, 338–348 (2021).
Qu, M., Qin, G., Fan, J., Du, A. & Sun, Q. Boron-rich boron nitride nanomaterials as efficient metal-free catalysts for converting CO2 into valuable fuel. Appl. Surf. Sci. 555, 149652 (2021).
Wang, Y. et al. Openmouthed β-SiC hollow-sphere with highly photocatalytic activity for reduction of CO2 with H2O. Appl. Catal., B 206, 159–167 (2017).
Xu, G. et al. Integrating the g-C3N4 nanosheet with B–H bonding decorated metal–organic framework for CO2 activation and photoreduction. ACS Nano 12, 5333–5340 (2018).
Cao, Y. et al. B–O bonds in ultrathin boron nitride nanosheets to promote photocatalytic carbon dioxide conversion. ACS Appl. Mater. Interfaces 12, 9935–9943 (2020).
Chen, P. et al. Rare-earth single-atom La–N charge-transfer bridge on carbon nitride for highly efficient and selective photocatalytic CO2 reduction. ACS Nano 14, 15841–15852 (2020).
Feng, H. et al. Surface nonpolarizationof g-C3N4 by decoration with sensitized quantum dots for improved CO2 photoreduction. ChemSusChem 11, 4256–4261 (2018).
Zhang, Y., Xia, B., Ran, J., Davey, K. & Qiao, S. Z. Atomic-level reactive sites for semiconductor-based photocatalytic CO2 reduction. Adv. Energy Mater. 10, 1903879 (2020).
Cao, Y. et al. Modulating electron density of vacancy site by single Au atom for effective CO2 photoreduction. Nat. Commun. 12, 1675 (2021).
He, Y. et al. 3D hierarchical ZnIn2S4 nanosheets with rich Zn vacancies boosting photocatalytic CO2 reduction. Adv. Funct. Mater. 29, 1905153 (2019).
Wang, J. et al. Effect of S vacancy in Cu3SnS4 on high selectivity and activity of photocatalytic CO2 reduction. Appl. Catal., B 297, 120498 (2021).
Li, X. et al. Selective visible-light-driven photocatalytic CO2 reduction to CH4 mediated by atomically thin CuIn5S8 layers. Nat. Energy 4, 690–699 (2019).
Zhu, X. et al. Unique dual-sites boosting overall CO2 photoconversion by hierarchical electron harvesters. Small 17, 2103796 (2021).
Bae, K. L., Kim, J., Lim, C. K., Nam, K. M. & Song, H. Colloidal zinc oxide-copper(I) oxide nanocatalysts for selective aqueous photocatalytic carbon dioxide conversion into methane. Nat. Commun. 8, 1156 (2017).
Deng, Y. et al. Synergy effect between facet and zero-valent copper for selectivity photocatalytic methane formation from CO2. ACS Catal. 12, 4526–4533 (2022).
Horani, F. & Lifshitz, E. Unraveling the growth mechanism forming stable γ-In2S3 and β-In2S3 colloidal nanoplatelets. Chem. Mater. 31, 1784–1793 (2019).
Rangappa, A. P. et al. In situ growth of Ag2S quantum dots on SnS2 nanosheets with enhanced charge separation efficiency and CO2 reduction performance. J. Mater. Chem. A 10, 7291–7299 (2022).
Liu, Y., Liu, M. & Swihart, M. T. Reversible crystal phase interconversion between covellite CuS and high chalcocite Cu2S nanocrystals. Chem. Mater. 29, 4783–4791 (2017).
Zhang, Y. et al. Photocatalytic CO2 reduction: identification and elimination of false-positive results. ACS Energy Lett. 7, 1611–1617 (2022).
Weng, B., Qi, M. Y., Han, C., Tang, Z. R. & Xu, Y. J. Photocorrosion inhibition of semiconductor-based photocatalysts: basic principle, current development, and future perspective. ACS Catal. 9, 4642–4687 (2019).
Yu, H., Huang, X., Wang, P. & Yu, J. Enhanced photoinduced stability and photocatalytic activity of CdS by dual amorphous cocatalysts: synergistic effect of Ti(IV)-hole cocatalyst and Ni(II)-electron cocatalyst. J. Phys. Chem. C. 120, 3722–3730 (2016).
Netzsch, P., Pielnhofer, F. & Höppe, H. A. From S–O–S to B–O–S to B–O–B bridges: Ba[B(S2O7)2]2 as a model system for the structural diversity in borosulfate chemistry. Inorg. Chem. 59, 15180–15188 (2020).
Xiong, X. et al. Photocatalytic CO2 reduction to CO over Ni single atoms supported on defect-rich zirconia. Adv. Energy Mater. 10, 2002928 (2020).
Wang, Y. et al. Direct and indirect Z-scheme heterostructure-coupled photosystem enabling cooperation of CO2 reduction and H2O oxidation. Nat. Commun. 11, 3043 (2020).
He, Y. et al. Quantitative evaluation of carrier dynamics in full-spectrum responsive metallic ZnIn2S4 with indium vacancies for boosting photocatalytic CO2 reduction. Nano Lett. 22, 4970–4978 (2022).
Caminiti, R., D’Ilario, L., Martinelli, A., Piozzi, A. & Sadun, C. DSC, FT-IR, and energy dispersive X-ray diffraction applied to the study of the glass transition of poly(p-phenylene sulfide). Macromolecules 30, 7970–7976 (1997).
Yu, X. et al. Lithium storage in conductive sulfur-containing polymers. J. Electroanal. Chem. 573, 121–128 (2004).
Wang, X. et al. Sulfurized polyacrylonitrile cathodes with high compatibility in both ether and carbonate electrolytes for ultrastable lithium–sulfur batteries. Adv. Funct. Mater. 29, 19029029 (2019).
Xu, C. et al. Photothermal coupling factor achieving CO2 reduction based on palladium-nanoparticle-loaded TiO2. ACS Catal. 8, 6582–6583 (2018).
Xu, J. et al. Efficient infrared-light-driven CO2 reduction over ultrathin metallic Ni-doped CoS2 nanosheets. Angew. Chem. Int. Ed. 60, 8705–8709 (2021).
Jiao, X. et al. Partially oxidized SnS2 atomic layers achieving efficient visible-light-driven CO2 reduction. J. Am. Chem. Soc. 139, 18044–18051 (2017).
Li, J. et al. Interfacial engineering of Bi19Br3S27 nanowires promotes metallic photocatalytic CO2 reduction activity under near-infrared light irradiation. J. Am. Chem. Soc. 143, 6551–6559 (2021).
Tang, W., Sanville, E. & Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Mat. 21, 084204 (2009).
Sanville, E., Kenny, S. D., Smith, R. & Henkelman, G. Improved grid-based algorithm for bader charge allocation. J. Comput. Chem. 28, 899–980 (2007).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grants no. 21972020, Z.Z.), and the Natural Science Foundation of Fujian Province of P. R. China (2020L3003, Z.Z.).
Author information
Authors and Affiliations
Contributions
X.W. and Z.Z. conceived the concept and supervised the research. Y.K., W.L., and J.S. performed the DFT calculations. Y.C. and M.L. performed the experimental work. J.L., R.Y., and W.D. helped to write—review & edit the manuscript. All authors designed the experiments, analyzed the data, and drafted the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Tao Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chai, Y., Kong, Y., Lin, M. et al. Metal to non-metal sites of metallic sulfides switching products from CO to CH4 for photocatalytic CO2 reduction. Nat Commun 14, 6168 (2023). https://doi.org/10.1038/s41467-023-41943-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-023-41943-x
This article is cited by
-
A review of photocatalytic CO2 reduction: exploring sustainable carbon emission mitigation from thermodynamics to kinetics and strategies for enhanced efficiency
Journal of the Korean Ceramic Society (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.