Abstract
In heterogeneous catalysis, the interface between active metal and support plays a key role in catalyzing various reactions. Specially, the synergistic effect between active metals and oxygen vacancies on support can greatly promote catalytic efficiency. However, the construction of high-density metal-vacancy synergistic sites on catalyst surface is very challenging. In this work, isolated Pt atoms are first deposited onto a very thin-layer of MoO3 surface stabilized on γ-Mo2N. Subsequently, the Pt–MoOx/γ-Mo2N catalyst, containing abundant Pt cluster-oxygen vacancy (Ptn–Ov) sites, is in situ constructed. This catalyst exhibits an unmatched activity and excellent stability in the reverse water-gas shift (RWGS) reaction at low temperature (300 °C). Systematic in situ characterizations illustrate that the MoO3 structure on the γ-Mo2N surface can be easily reduced into MoOx (2 < x < 3), followed by the creation of sufficient oxygen vacancies. The Pt atoms are bonded with oxygen atoms of MoOx, and stable Pt clusters are formed. These high-density Ptn–Ov active sites greatly promote the catalytic activity. This strategy of constructing metal-vacancy synergistic sites provides valuable insights for developing efficient supported catalysts.
Similar content being viewed by others
Introduction
The design of efficient supported catalysts has been a frontier research in catalysis. For heterogeneous catalytic reactions, due to the complexity and diversity of reactant molecules, high-performance supported catalysts always require the cooperation of multiple components1,2,3,4. At the metal-support interface, the active metal and the support work collectively to activate different reactant molecules to form active intermediates, which can greatly facilitate a variety of important catalytic reactions, such as CO oxidation5, water–gas shift reaction6, and CO2 reduction7. Therefore, the construction of active interfaces is essential for the design of efficient supported catalysts. However, the catalytic properties of interfacial structure can be influenced by many factors, such as the size of active metals1,8, the type of supports9, the strength of metal-support interactions10, making targeted creation of active interfaces with specific structure a challenge.
For reducible supports, the oxygen vacancy on surfaces has attracted extensive attention in the field of catalysis11,12,13,14,15,16. Importantly, interfacial oxygen vacancies can form special active sites in cooperation with neighboring active metals, promoting catalytic reactions effectively. Recently, the synergistic effect of active metals and vacancies has been demonstrated5,6,17. For active metal-oxygen vacancy synergistic site, the oxygen vacancy plays a crucial role in anchoring and activating the active metal, thus stabilizing interfacial structures and accelerating the activation of reactant molecules. So far, research works on the synergistic effect between active metal and oxygen vacancy have been mostly focused on the catalysts using reducible oxides as support. However, due to the stability of the surface oxygen atoms, the ability to generate surface oxygen vacancies in these oxides has been restricted18,19,20, which undoubtedly limits the creation of active metal-oxygen vacancy synergistic sites. Besides oxide supports, transition-metal nitrides like Mo2N have received extensive attention in a variety of catalytic reactions due to their unique electronic structure9. Due to the contraction of the d-band caused by the interstitial incorporation of N in the Mo metal lattice, Mo2N exhibited a noble-metal-like electronic structure. In order to prevent the spontaneous combustion of molybdenum nitrides in air, it is often necessary to slowly oxidize its surface during the preparation process. Under the effect of surface heterostructural stress, the molybdenum oxide surface was easily reduced21, providing the potential to solve the problem of limited oxygen vacancy concentration on the surfaces of conventional oxides.
The reverse water‒gas shift (RWGS) reaction is an important reaction to utilize CO222,23,24,25. The product CO can be further converted into high-value chemicals through Fischer‒Tropsch synthesis26,27,28. However, CO2 molecule is very stable, and the RWGS reaction is endothermic, making the conversion of CO2 at low temperature very challenging. In general, for the RWGS catalysts, active metal clusters can dissociate H2, while the oxygen vacancies can activate CO2. Therefore, the creation of catalysts with high-density synergistic sites between active metal cluster and oxygen vacancy may greatly improve the low-temperature activity of catalysts. Herein, we deposited single Pt atoms on the γ-Mo2N support with a MoO3 passivation surface. Based on the stress of MoO3/γ-Mo2N heterostructure, the surface MoO3 was transformed into MoOx structure with the creation of sufficient surface oxygen vacancies in the pretreatment and reaction processes. Meanwhile, isolated Pt atoms were reduced to form Pt clusters with the size less than 1 nm, which were anchored on the MoOx surface to form abundant and stable metal cluster-oxygen vacancy synergistic sites. The obtained Pt–MoOx/γ-Mo2N catalyst exhibited unmatched catalytic performance with a CO yield of 17.2 × 10−5 mol gcat‒1 s‒1 at 300 °C, to catalyze the low-temperature reverse water–gas shift (RWGS) reaction, which far exceeded those of previously reported catalysts. Comprehensive in situ characterizations and theoretical calculations revealed that the Ptn–Ov synergistic sites enhanced the activation of reactant molecules. This strategy of constructing high-density active metal-vacancy interfacial sites using the stress of heterostructure and metal‒support interactions provides a new way to enhance the activity of supported catalysts.
Results
Structure characterization of the Pt–MoO3/γ-Mo2N catalyst
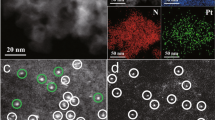
The molybdenum oxide structure on the γ-Mo2N surface was the safeguard against spontaneous combustion of γ-Mo2N in air. During the preparation of γ-Mo2N sheet, the surface of γ-Mo2N was slightly oxidized by passivation treatment with low concentration of oxygen (1% O2/Ar) at room temperature to form the special MoO3/γ-Mo2N heterostructure. The stress of MoO3/γ-Mo2N heterostructure made MoO3 highly susceptible to deoxygenation to form MoOx (2 < x < 3) surface with rich oxygen vacancies21. After the loading of Pt, the MoOx surface provided the possibility for the construction of high density Pt-oxygen vacancy sites. In order to verify that the as-prepared catalyst had the expected structure as shown in Fig. 1a, comprehensive characterizations were carried out. The transmission electron micrograph (TEM) images exhibited that all fresh and used catalysts had sheet structures (Supplementary Fig. 1). To explore the morphology and particle size of catalysts, high-angle annular dark-field scanning TEM measurements (HAADF-STEM) were conducted. For fresh 0.5Pt–MoO3–Mo2N, the porous γ-Mo2N nanosheet was composed of numerous Mo2N particles with diameters ranging from 3 to 5 nm, and isolated Pt atoms were anchored on the γ-Mo2N support (Fig. 1b and Supplementary Fig. 2). As illustrated in Fig. 1c and Supplementary Fig. 3, most of the Pt atoms with Pt loading of 0.59 wt.% (Supplementary Table 1) were transformed to Pt clusters with diameters less than 1 nm after the RWGS reaction, suggesting the aggregation of Pt atoms during the reduction pretreatment. The element mapping results indicated the weak surface oxidation of Mo2N, with O signal appearing neared the Mo and N elements (Supplementary Fig. 4). The HAADF-STEM results of fresh and used 0.5Pt–MoO3/γ-Mo2N catalysts indicated that the bulk structure of γ-Mo2N support was stable during the reaction. As shown in Supplementary Figs. 5a and 6, for both the fresh and used catalysts, only X-ray diffraction (XRD) patterns of γ-Mo2N were detected without any discernible peak of Pt species, implying that Pt species were highly dispersed on the support. However, different from the XRD results, Raman spectra of the samples before and after the RWGS reaction only showed the signal of MoO321. Thus, the surface of the catalyst was covered with a thin layer of MoO3 passivation structure in the air (Supplementary Figs. 5b and 7), which was consistent with the element mapping result. Furthermore, the in situ Raman study of 0.5Pt–MoO3/γ-Mo2N illustrated that the surface MoO3 was transformed to MoOx by deoxygenation in the RWGS reaction (Supplementary Fig. 5b), accompanied by the formation of abundant oxygen vacancies.
In order to explore the local structure of Pt–MoO3/γ-Mo2N, X-ray absorption fine structure (XAFS) spectra were measured. As shown in Fig. 1d, the extended X-ray absorption fine structure (EXAFS) spectra showed only one peak at ~1.7 Å as the Pt-O contribution in fresh 0.5Pt–MoO3/γ-Mo2N, while the peak at ~2.6 Å from Pt–Pt contribution was dominant in the used sample. Thus, the single Pt atoms in 0.5Pt-MoO3/γ-Mo2N agglomerated into nanoclusters during the RWGS reaction, which was consistent with the HAADF-STEM results. For the Pt-MoO3/γ-Mo2N catalysts with higher Pt loadings (1 wt. % and 2 wt. %) before and after the reaction, in addition to the Pt–O coordination, Pt–Pt bond was observed, suggesting the presence of Pt clusters (Supplementary Fig. 8). After the reaction, the Pt–Pt contribution increased, indicating the reduction of Pt single atoms (Supplementary Figs. 8 and 9). Meanwhile, the small coordination numbers of Pt–Pt bond (≤3.0) in all samples suggested that Pt species were highly dispersed on the support without obvious aggregation into nanoparticles (Supplementary Fig. 10 and Supplementary Table 2). The presence of Pt–O peak indicated that Pt was bound with O atoms rather than Mo atoms. Supplementary Fig. 11 showed the X-ray absorption near edge spectra (XANES) of fresh Pt–MoO3/γ-Mo2N catalysts. The white line intensities in the spectra of all catalysts were between that of PtO2 and Pt foil, suggesting that Pt species were positively charged. With the increasing of Pt loading, more metallic Pt was detected. The XANES spectra of Pt–MoO3/γ-Mo2N (Fig. 1e) showed that the oxidized Pt species were partially reduced during the RWGS reaction, which is consistent with the EXAFS results. Based on the above results, we proposed the following structure of catalyst (Fig. 1a): a thin layer of MoOx with abundant oxygen vacancies was in situ formed from MoO3 on the surface of the γ-Mo2N, with Pt clusters anchored on MoOx. The MoOx with abundant oxygen vacancies provided a platform for the construction of sufficient platinum cluster-oxygen vacancy synergistic sites.
Catalytic performance of the Pt–MoOx/γ-Mo2N catalysts in the RWGS reaction
The catalytic performances of the Pt–MoOx/γ-Mo2N catalysts for the RWGS reaction were evaluated at various temperatures under a very high space velocity of 300,000 mL gcat‒1 h‒1. As illustrated in Fig. 2a, the pure γ-Mo2N support was fully inactive at relatively low temperatures (<300 °C), indicating that the MoO3/γ-Mo2N support without platinum clusters could not catalyze this reaction. The deposition of Pt on MoO3/γ-Mo2N effectively promoted the activity of catalysts at low temperatures (Fig. 2a and Supplementary Fig. 12). With the increasing of Pt loading, the catalytic efficiency increased gradually. CO2 could even be converted at 200 °C when the Pt loading reached 0.5 wt.%. When the Pt loading exceeded 0.5 wt.%, the CO2 conversion did not increase much. At 300 °C, the CO2 conversion rate of 0.5Pt–MoOx/Mo2N was close to the upper limit of thermodynamic equilibrium, which was much higher than that of the reference 2Pt–CeO2 catalyst with 2 wt.% Pt loading (Fig. 2a). Importantly, the reaction rate of 0.5Pt–MoOx/γ-Mo2N reached as high as 17.2 × 10−5 molCO2 gcat‒1 s‒1 at 300 °C, which was definitely the highest value compared with all other reported catalysts for low-temperature RWGS reactions. This value even exceeded those of many catalysts working under much higher temperature (350‒500 °C) (Fig. 2b and Supplementary Table 3). Obviously, the Pt–MoOx/γ-Mo2N catalyst improved the catalytic efficiency for low-temperature RWGS reactions to a new level. Furthermore, 0.5Pt–MoOx/γ-Mo2N showed good stability, and it could maintain ~80% initial CO2 conversion after 300 h reaction at 300 °C with a space velocity of 300,000 mL gcat−1 h−1 (Fig. 2e). Besides, the apparent activation energy (Ea) for all Pt–MoOx/Mo2N catalysts was around 40 kJ mol‒1 (Supplementary Fig. 13), which was only half than that of 2Pt–CeO2 (~82 kJ mol−1), indicating the great catalytic efficiency of Pt-MoOx/Mo2N (Fig. 2c). As shown in Fig. 2d, the reaction orders of H2 were 0.43, 0.55 and 0.61 at 250, 280, and 300 °C, respectively. The reaction orders of CO2 were 0.07, 0.20, and 0.26 at 250, 280, and 300 °C, respectively. The reaction orders of CO2 and H2 increased with rising temperature, suggesting that the adsorption of reactant molecules became difficult with rising temperature. Meanwhile, the low CO2 reaction order meant that CO2 was activated very easily on catalytic surfaces. The mass transfer limitation of Pt–MoOx/Mo2N was excluded to ensure the accuracy of kinetic data (Supplementary Fig. 14).
a CO2 conversion over different catalysts at various temperatures. b The mass specific activities of 0.5Pt–MoOx/Mo2N and other typical catalysts that were used in RWGS reaction. (1) Cu/β-Mo2C (ref. 36.); (2) Cu–Zn–Al (ref. 36.); (3) Pt/CeO2 (ref. 37.); (4) AuMo/SiO2 (ref. 38.); (5) In2O3–CeO2 (ref. 39.); (6) NiAu/SiO2 (ref. 40.); (7) Pt–CeO2 (ref. 41.); (8) TiO2/Cu (ref. 42.); (9) SiO2/Cu (ref. 42.); (10) Pt-TiO2 (ref. 43.); (11) Rh@S-1 (ref. 44.); (12) Ni-in-Cu (ref. 45.); (13) NiAu/SiO2 (ref. 40.); (14) K80–Pt–L (ref. 46.); (15) K200–Pt–L (ref. 46.); (16) In2O3–CeO2 (ref. 39.). c Apparent activation energy (Ea) of different catalysts. d Kinetic orders of reactants (CO2 and H2) for 0.5Pt–MoOx–Mo2N at various temperatures. e Long-term stability test of 0.5Pt–MoOx–Mo2N.
Surface structure of catalyst under the RWGS reaction conditions
The deposition of Pt greatly improved the activity of catalyst, so it was important to reveal the local structure and electronic structure of Pt species in the actual reaction process. Because the very low loading of Pt (0.5 wt.%) in 0.5Pt–MoOx/Mo2N could cause the poor ratio of signal to noise, we conducted the XAFS measurements over 1Pt–MoOx/Mo2N (1.0 wt.%). During the H2 pretreatment, the XANES profile at the Pt L3 edge revealed a gradual decrease of the white line (feature around 11570 eV) in the first 1 h (Fig. 3a), indicating that highly oxidized Pt species were gradually reduced. However, after H2 pretreatment for 2 h, the Pt could still maintain partially oxidized state. Besides, in the subsequent RWGS reaction, the partially oxidized state of Pt remained stable (Fig. 3b), suggesting the strong electron interaction between Pt and the support. The average coordination number of Pt–Pt bonds in the reaction process was ~2.5, indicating that the Pt was highly dispersed on the support in the form of clusters. Meanwhile, there was a Pt–O contribution with an average coordination number of 1.8, which originated from the bonding interaction between Pt and MoOx (Fig. 3c). Because the catalytic properties of 1Pt–MoOx/Mo2N were similar to those of the 0.5Pt–MoOx/Mo2N sample, it could be inferred that Pt in 0.5Pt–MoOx/Mo2N existed as clusters with smaller size, which was in good agreement with the HAADF images of the used catalyst (Fig. 1c and Supplementary Fig. 3).
In situ XANES profile at Pt L3 edge under a H2 pretreatment and b RWGS reaction, respectively. c The change of Pt coordination number during the RWGS reaction. d In situ Raman spectra of 0.5Pt–MoOx/Mo2N under various conditions. e XPS spectra of fresh 0.5Pt–MoO3/Mo2N and quasi in situ XPS spectra of 0.5Pt–MoOx/Mo2N after the treatment of 5% H2/Ar and 23% CO2/69% H2/Ar at 300 °C for 30 min.
Raman spectra clearly exhibited the surface structure of support in the RWGS reaction. From the ex situ Raman results of the fresh and used catalysts (Supplementary Fig. 7), there was a thin layer of MoO3 on the catalyst surface. In order to explore the surface structure of support under the actual RWGS reaction conditions, systematic in situ Raman spectra were studied. As shown in Supplementary Fig. 15a, when 0.5Pt–MoOx/Mo2N was treated by 5% H2/Ar at room temperature (RT), MoO3 was transformed into MoOx in the reduction process. The loss of oxygen implied the generation of abundant surface oxygen vacancies. With the rising of test temperature under 5% H2/Ar, the characteristic peaks of MoOx remained stable. Furthermore, the in situ Raman spectra of 0.5Pt–MoOx/Mo2N under the RWGS reaction were measured. The surface structure of catalyst was MoOx under the actual reaction process (Supplementary Fig. 15b). Further, during the 10 h RWGS reaction, the signal of MoOx had no obvious change, suggesting that the MoOx was very stable (Supplementary Fig. 16). For the γ-Mo2N support, the in situ Raman result (Supplementary Fig. 17) indicated that without the assistance of Pt species, the MoO3 surface on the γ-Mo2N was easily reduced to form MoOx. However, for pure MoO3, there was no signal of MoOx in the RWGS reaction process (Supplementary Fig. 18a), suggesting that the reduction of MoO3 occurred only at the surface of the heterostructured MoO3/γ-Mo2N. The stress of MoO3/γ-Mo2N heterostructure was the key to reduce MoO3. The MoOx with plenty of oxygen vacancies on the Mo2N surface undoubtedly provided a guarantee for the formation of sufficient Ptn–Ov sites.
We further used quasi in situ XPS spectra to get the information on the surface structure transformation of Pt–MoOx/Mo2N under various atmospheres (Fig. 3e). In all XPS spectra under different atmospheres, the Mo 3d spectra could be adapted to three doublets. The predominant doublet at 228.8 and 231.9 eV was attributed to the Mo–N bond29. The peaks at 229.4 and 232.6 eV were ascribed to Mo4+. The other doublet at 232.1 and 234.9 eV was attributed to Mo6+. Compared with the fresh catalyst, after the pretreatment and the RWGS reaction, the content of oxygen atoms on catalyst surfaces decreased from 23.2 to 14.3% and 13.6%, respectively, indicating that ~40 % of oxygen atoms were reduced to form vacancies. As shown in Fig. 3d, after the treatment by NH3, the Raman peaks of MoOx existed in the RWGS reaction process disappeared, which suggested the nitridation of MoOx structure. After that, under the effect of H2O, the MoOx structure was regenerated. However, as shown in Supplementary Fig. 19, the broken MoOx structure could not be regenerated in the RWGS reaction, indicating that the H2O generated in the RWGS reaction could leave the catalyst surface quickly without oxidizing the catalyst surface. Besides, the color change of the MoO3 sample treated with NH3 flow and the quasi in situ XPS spectra of 0.5Pt–MoOx/Mo2N (Supplementary Figs. 20 and 21) further confirmed that the NH3 flow converted a part of the oxide into Mo2N. Furthermore, when the catalyst was treated by NH3 at 650 °C for 30 min (Supplementary Fig. 21b), the catalyst surface was not fully nitrided into Mo2N. Thus, it was difficult to completely remove the Mo-O structure on the catalyst surface. The above series of in situ characterizations showed that the catalyst surface was Pt–MoOx under the RWGS reaction.
Ptn–Ov synergistic effect
We have found that the nitrogenation of the MoOx by NH3 undoubtedly caused the catalyst deactivation (Fig. 4a). Meanwhile, the MoOx/γ-Mo2N sample without Pt deposition exhibited no activity even at 300 °C (Fig. 2a). So the excellent activity for the RWGS reaction was the result of the synergistic effect of oxygen vacancies and Pt clusters. CO2 activation was a prerequisite for CO2 hydrogenation. Hence, it was of great significance to explore how CO2 was activated. The CO2-TPD result demonstrated that the addition of Pt effectively enhanced the adsorption of CO2 (Fig. 4b). Theoretical simulations showed that the CO2 molecule exhibited obvious activation upon the adsorption on the Pt4-MoOx surface model, which was reflected in the bending of molecular configuration and the acquisition of electric charge30,31. In Fig. 4c and Supplementary Fig. 22a, b, we presented three adsorption structures of CO2 (denoted as CO2/Pt4–MoOx-I, CO2/Pt4–MoOx-II, and CO2/Pt4–MoOx-III, respectively), whose energy were nearly degenerated with an energy difference of less than 0.1 eV. Among the three of them, the CO2 molecule in CO2/Pt4–MoOx-I had the largest angular bending (from 180.0° to 118.8° vs to 133.3° and 148.5°) and the maximum charge transfer (−0.63 e vs −0.53 e and −0.22 e), indicating that it was mostly activated. Thus, we used CO2/Pt4–MoOx-I as a representative configuration for subsequent analysis. Projected electronic density of states (Fig. 4d) revealed that CO2 had effective interactions with surrounding atoms, especially Pt-1 and Pt-4 (Fig. 4c), and the corresponding differential charge density was shown in Fig. 4e. For comparison, the adsorption of CO2 was weak on both MoOx (without Pt) and Pt4–MoO3 (without oxygen vacancies) surface models, and the adsorbed CO2 molecule exhibited a linear configuration (Supplementary Fig. 22c, d). Hence, the coexistence of Pt and oxygen vacancies was very important for the activation of the CO2 reactant, consistent with the observed poor activity of the MoOx–Mo2N catalyst and also that of the 0.5Pt–MoOx/Mo2N catalyst pretreated by oxygen (Supplementary Fig. 23). Both experimental and simulation results indicated that the Ptn–Ov synergistic effect promoted the catalytic performance in the RWGS reaction.
a The RWGS reaction rate of 0.5Pt–MoOx/Mo2N after different pretreatments. b CO2-TPD of MoOx/Mo2N and 0.5Pt–MoOx/Mo2N. c Adsorption structure of CO2 on the Pt4–MoOx surface model (denoted as CO2/Pt4–MoOx-I). The other two adsorption configurations (denoted as CO2/Pt4–MoOx-II and CO2/Pt4–MoOx-III) are shown in Supplementary Fig. 22a, b. d Projected electronic density of states (PDOS) of the CO2 adsorbate, d-orbitals of the two Pt atoms (Pt-1 and Pt-4), and d-orbitals of the Mo-3 atom in CO2/Pt4–MoOx-I. e Electron transfer between adsorbed CO2 and Pt4–MoOx surface according to the differential charge density analysis. The blue and red colors represent the electron accumulation and depletion, respectively. f Energy profiles of the three reaction routes (redox, carboxyl, and formate) on Pt4–MoOx, depicted in gray, red, and cyan, respectively. The black line represents the common parts of the three pathways. The configurations of intermediates and transition states (TS) in the carboxyl route (energetically the most favorable according to the simulations) are displayed at the bottom, while those in the other two pathways (redox and formate) are shown in Supplementary Figs. 25 and 26, respectively.
We further explored the reaction mechanism and the influence of the synergistic effect between oxygen vacancies and Pt clusters on reaction pathways. For the RWGS reaction, the reaction mechanisms have been divided into two types, redox mechanism, and associative mechanism32. The difference between these two mechanisms lies in whether or not the dissociated H was involved in the formation of active intermediates33. The dissociation experiment of CO2 indicated that CO2 itself could not dissociate to CO without the assistance of H2 (Supplementary Fig. 24a). And as shown in Supplementary Fig. 24b, under the effect of H2, the signal intensity of CO2 decreased from ~150 °C, accompanied with the formation of CO, suggesting that H2 was necessary for the reaction process34. A similar result was obtained by the in situ diffused reflectance infrared Fourier transform spectroscopy (DRIFTS) spectra. As illustrated in Supplementary Fig. 24c, when the catalyst was treated with CO2 at 300 °C, no CO gaseous signal appeared. However, in the presence of CO2 and H2, the distinct gaseous signal of CO was observed (Supplementary Fig. 24d), indicating that H2 was involved in the formation of active intermediates. Thus, the reaction catalyzed by 0.5Pt–MoOx/Mo2N followed the associative mechanism. Computational simulations were performed to investigate reaction pathways, as shown in Fig. 4f. Here, both the redox and the associative (including the carboxyl and the formate routes) mechanisms were considered (Fig. 4f). The redox mechanism would not appear in the reaction process (Supplementary Fig. 25), since the corresponding highest energy barrier (2.00 eV in TS1) was much higher than that of the associative mechanism (1.03 eV in TS3 from the carboxyl route). This was consistent with the experiments that CO2 could not dissociate by itself. Regarding the associative mechanism, it was found that Pt atoms and oxygen vacancies were both involved in the reactions. The adsorption and activation of CO2 was the prerequisite for the processing of RWGS reaction. Computational simulations indicated that Pt cluster or oxygen vacancy alone could not effectively adsorb and activate CO2 (Supplementary Fig. 22c, d). However, CO2 could be easily activated at the interface between Pt cluster and oxygen vacancy (Fig. 4c and Supplementary Fig. 22a, b). The activation of CO2 at the interface between Pt cluster and oxygen vacancy guaranteed the further formation of reactive intermediate. Besides, the intermediates in the reaction pathways, like COOH* and HCOO*, were connected with Pt and an adjacent unsaturated Mo atom that appeared upon the removal of oxygen atoms. These results further supported the synergistic effect of Pt clusters and oxygen vacancies in the catalytic process. Our calculations showed that the energy barrier in the carboxyl route (1.03 eV in TS3) was lower than the value (1.81 eV in TS3) in the formate one, suggesting that the carboxyl route was energetically more favorable (Fig. 4f and Supplementary Fig. 26). We noted in passing that from our experiments, we could not determine whether the actual reaction process was via the carboxyl route or the formate pathway (But no matter which of the two was adopted, the cooperation of Pt clusters and oxygen vacancy would always play a crucial role in promoting the RWGS reaction).
Discussion
Synergizing multiple catalytic sites is the key to enhance the efficiency of catalysts in heterogeneous catalysis. In this work, using the stress of MoO3/γ-Mo2N structure and the interaction between Pt and support, efficient catalysts with high density of Ptn–Ov synergistic sites were successfully constructed. The Pt–MoOx/Mo2N catalyst showed excellent activity and solid stability in low-temperature RWGS reactions, which was significantly better than other reported catalysts. During the pretreatment and reaction process, surface MoO3 was transformed into MoOx with the creation of a large number of oxygen vacancies. Through in situ reduction, isolated Pt atoms were converted to Pt clusters. Highly dispersed Pt clusters were stably bonded with the O atoms of MoOx, in which the interaction between Pt and MoOx prevented the Pt clusters from sintering. By the combination of experimental investigations and theoretical calculations, the synergistic catalytic effect between Pt clusters and oxygen vacancies was proved to be the key to promote the reaction. This work provides an example to explore the interfacial structure of catalysts in the actual reaction process, and paves a new way to amplify the synergistic effect of active metal and support by taking the advantages of heterostructure.
Methods
Preparation of MoO3
The MoO3 Precursor was prepared by hydrothermal method. Firstly, 1 g of P123 was dissolved by 40 mL deionized water in a Teflon bottle. Then, 0.9 g of Na2MoO4·H2O and 5 mL deionized water were added onto the above suspension to form a stock solution. Next, 3 mL of concentrated hydrochloric acid (37 wt.%) was added in drops to the stock solution. Finally, the Teflon bottle was sealed into a stainless autoclave tightly and heated at 100 °C for 12 h in the oven. The resulting precipitates are collected by centrifugation and washed with deionized water and absolute ethanol, followed by drying in the oven at 70 °C for 10 h. The resulting solid is heated in tube furnace at 400 °C for 4 h to obtain the MoO3 powder. For the preparation of γ-Mo2N support, the above MoO3 powder was ground and transferred into a quartz tube and ammonized by pure NH3 (40 mL/min) at 650 °C for 4 h. After cooling, the prepared γ-Mo2N was passivated with 1% O2/Ar mixed gas for 2 h. The 1% O2/Ar mixed gas could oxide the surface of the fresh γ-Mo2N to prevent the full oxidation of the bulk-phase γ-Mo2N.
Preparation of Pt–MoO3/Mo2N catalysts
A series of Pt–MoO3–Mo2N catalysts were prepared by the ethylene glycol method. Firstly, the γ-Mo2N support (400 mg) and a designed amount of chloroplatinic acid (0.19 mol/L, 54 µL) were added into a beaker containing 100 mL ethylene glycol, and then stirred for 0.5 h and ultrasonicated for another one hour to achieve good dispersion of the slurry. Next, adjust the pH of the slurry to 1.5 with HCl aqueous solution (6.2 wt. %). After that, the slurry was refluxed at 140 °C for 2 h. The resulting precipitates are collected by centrifugation and washed with deionized water and absolute ethyl alcohol, followed by drying in the oven at 40 °C to obtain the fresh catalysts. In this work, the obtained catalysts were nominated as xPt–MoO3/Mo2N (x = 0.2, 0.5, 1 and 2), where x is the platinum content in weight percent (x = [Pt/Mo2N] wt× 100%).
Preparation of reference Catalyst (Pt–CeO2)
The Pt–CeO2 catalyst was synthesized by deposition–precipitation method, according to the previous reports35. 0.5 g CeO2 nanorod support was dispersed into 30 mL ultrapure water. A certain amount of chloroplatinic acid solution (0.19 mol/L, 68 µL) was then added to the obtained suspension drop by drop and the pH of the solution was kept at 9 with the assistance of sodium carbonate solution (0.1 mol/L) during the whole process. The resulting precipitate was aged at room temperature for 1 h, and washed by ultrapure water. The solid was then dried at 70 °C for 10 h in the oven and calcined at 400 °C for 4 h in muffle furnace.
X‒ray diffraction (XRD)
For the ex situ XRD data, all experiments were operated on PANalytical X’pert3 powder diffractometer with CuK radiation (λ = 0.15406 nm).
Transmission electron microscope (TEM)
TEM was conducted by JEM-2100F (JEOL) instrument operating at 200 kV. The samples were sonicated in ethanol and being dropped on the carbon-coated Cu grid before test. The High-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) images were obtained on a Thermo Scientific Themis Z microscope equipped with a probe-forming spherical-aberration corrector.
Inductively coupled plasma-atomic emission spectroscopy (ICP-AES)
For 0.5Pt–MoO3/Mo2N catalyst, the ICP-AES measurement was carried out on an IRIS Intrepid II XSP instrument (Thermo Electron Corporation).
X-ray photoelectron spectroscopy (XPS)
Quasi in situ XPS experiments were carried out on a Thermo Scientific ESCALAB Xi+ XPS instrument. The spectrums of Mo 3d, C 1s, O 1s, and Pt 4f were obtained after the catalysts were pretreated by different atmospheres and temperatures for 1 h.
Raman spectroscopy
Ex situ and in situ Raman spectra were acquired on a Raman microscope system (HORIBA JY) with laser excitation at 473 nm. The integration times of Ex situ and in situ Raman spectra were 60 s and 5 min, respectively. For the in situ Raman, the micro-Raman reaction cell (Xiamen TOPS) equipped with a quartz window has a heating module that controls the test temperature. Five kinds of programs were conducted as follows.
-
(i)
The 0.5Pt–MoOx/Mo2N catalyst was pretreated by 5% H2/Ar at 300 °C for 30 min. After cooling to room temperature, the gas flow was switched to RWGS reaction gas (33% CO2, 67% H2), and then heated to 300 °C for 10 h. The Raman spectra was collected at 100, 200, and 300 °C, respectively.
-
(ii)
Firstly, the 0.5Pt–MoOx–Mo2N catalyst was treated with 5% H2/Ar at 300 °C for 30 min. Then, using the pure NH3 to purge the sample from room temperature and heated to 300 °C for 30 min. After that, the gas flow was changed to RWGS reaction gas (25% CO2, 75% H2) to treat the sample at 300 °C for 120 min. Finally, use ~3% H2O/Ar to purge the catalyst for another 30 min at 300 °C. The Raman spectra were collected under RWGS reaction gas, NH3, H2O/Ar at 300 °C, respectively.
-
(iii)
The whole test process was the same as the procedure in (ііі), except that the RWGS reaction gas (25% CO2, 75% H2) and pure NH3 were switched in a different order.
CO2 temperature-programmed desorption (CO2-TPD), CO2 dissociation experiment, temperature-programmed surface reaction (TPSR)
All these experiments were performed on a lab-made reactor and the outlet gases were analyzed by mass spectrum (LC-D200M, TILON). For these different three types of tests, the catalysts were firstly reduced in 5% H2/Ar at 300 °C for 30 min, and then flushed with Ar gas flow (30 mL min‒1) at room temperature for 30 min. For CO2-TPD, the catalysts were saturated with 2% CO2/Ar (30 mL min‒1) at room temperature for 30 min followed by purging with Ar gas flow (30 ml min‒1) for 30 min to purge all the physically adsorbed CO2 molecules on the surface of catalysts and the residual CO2 in the reaction tube. And then the CO2-TPD experiment was measured from room temperature to 300 °C with a ramping rate of 10 °C min‒1 under Ar gas flow (30 mL min−1). For CO2 dissociation experiment, after H2 activation and Ar gas purge, the samples were purged with 2% CO2/Ar with heating from room temperature to 300 °C. For TPSR, the samples were treated with mixed gas with 15% CO2 and 30% H2 with heating from room temperature to 300 °C.
In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS)
All of the in situ DRIFTS spectra were collected by using a Bruker Vertex 70 FTIR spectrometer with a mercury cadmium telluride (MCT) detector cooled with liquid nitrogen. The treatment process of CO2 on the 0.5Pt–MoOx–Mo2N catalyst was investigated by in situ DRIFTS measurement at 300 °C. Prior to the in situ DRIFTS measurement, ~30 mg sample was pretreated at 300 °C for 30 min under 5% H2/Ar mixed gas. The background spectra were collected under N2 atmosphere at 4 cm‒1 resolution at 300 °C. After the collection of the background spectrum, the mixed gas consisted of 2% CO2/Ar and was introduced into the chamber. Continuous recording of the IR profiles was maintained for 5 min. As for the test under RWGS reaction conditions, after background acquisition, the reaction gas with 15% CO2/30% H2/55% N2 is introduced into the in situ chamber. All DRIFTS results were analyzed by using OPUS software.
XAFS (X-ray absorption fine spectroscopy) experiments
XAFS was performed at the BL11B beamline in Shanghai Synchrotron Radiation Facility (SSRF, Energy 3.5 GeV, Current 250 mA in maximum, Si (111) double-crystals as double crystal monochromator which could cover the photon energy range from 4.5 to 18 KeV). The samples were measured in fluorescence mode, using a Lytle detector to collect the data.
XAFS of H2 reduction process: The XAFS sample was sealed in the reaction cell. The catalyst was pretreated by 5% H2/He at 300 °C for 2 h. The XAFS was continuously collected during the reduction process.
XAFS of the RWGS process: After the sample was reduced, the XAFS was collected under the reaction conditions.
All XAFS spectra were analyzed using the Ifeffit package version 1.2.11.
Catalytic measurements and kinetic tests
The activity of the catalysts for RWGS reaction was conducted by a fixed-bed reactor at atmospheric pressure. For temperature-dependant activity test, 10 mg sieved sample (20–40 mush) was mixed with 90 mg inert SiO2 and was packed into a quartz tube. Before the RWGS reaction, the sample was pretreated by 5% H2/Ar (30 mL/min) at 300 °C for 30 min. And then when the temperature of the catalyst dropped to room temperature, the gas flow was switched into RWGS reaction gas flow (23% CO2, 69% H2, 8% N2) with 50 mL/min. At each test temperature, the product was analyzed after 60 min of steady-state reaction. For the test of reaction rate, in order to obtain reaction rates in the kinetics region, appropriate amount of catalysts diluted with SiO2 were used and the CO2 conversion rate was controlled at a relative low level (<20%) by changing gas flow rate. The outlet product was analyzed by an on-line gas chromatograph equipped with a thermal conductivity detector (TCD). The gas flow rate was determined by the inner standard method, in which the N2 was used as the inner standard. The CO2 conversion and CO selectivity were calculated by the following equations:
where \({n}_{{{{{\rm{CO}}}}}_{2}}^{{{{\rm{in}}}}}\) is the concentration of CO2 in the reaction stream, and \({n}_{{{{{\rm{CO}}}}}_{2}}^{{{{\rm{out}}}}}\) is the concentration of CO2 in the outlet gas. \({A}_{{{{{\rm{CO}}}}}_{2}}^{{{{\rm{in}}}}}\) and \({A}_{{{{{\rm{N}}}}}_{2}}^{{{{\rm{in}}}}}\) refer to the chromatographic peak area of CO2 and N2 in the inlet gas, respectively, and \({A}_{{{{{\rm{CO}}}}}_{2}}^{{{{\rm{out}}}}}\) and \({A}_{{{{{\rm{N}}}}}_{2}}^{{{{\rm{out}}}}}\) refer to the chromatographic peak area of CO2 and N2 in the outlet gas, respectively. The chromatographic peak area of each component is proportional to the concentration of each component.
The selectivity of CO was calculated as
where \({n}_{{{{{{\rm{CO}}}}}}}^{{{{{{\rm{in}}}}}}}\) and \({n}_{{{{{{{\rm{CH}}}}}}}_{4}}^{{{{{{\rm{in}}}}}}}\)refer to the concentration of CO and CH4 in the outlet gas, respectively. \({f}_{{{{{{\rm{CO}}}}}}/{{{{{{\rm{N}}}}}}}_{2}}\) and \({f}_{{{{{{{\rm{CH}}}}}}}_{4}/{{{{{{\rm{N}}}}}}}_{2}}\) are relative correction factors of CO to N2 and CH4 to N2, respectively, which are determined by the calibrating gas. \({A}_{{{{{{\rm{CO}}}}}}}^{{{{{{\rm{out}}}}}}}\) and \({A}_{{{{{{{\rm{CH}}}}}}}_{4}}^{{{{{{\rm{out}}}}}}}\) are the chromatographic peak area of CO and CH4 detected by the TCD in the outlet gas.
The carbon balance was calculated as
where \({f}_{{{{{{{\rm{CO}}}}}}}_{2}/{{{{{{\rm{N}}}}}}}_{2}}\) is relative correction factor of CO2 to N2, which is determined by the calibrating gas. \({A}_{{{{{{{\rm{CO}}}}}}}_{2}}^{{{{{{\rm{out}}}}}}}\) is the chromatographic peak area of CO2 detected by the TCD in the outlet gas.
Apparent activation energy (Ea) and apparent kinetic orders
The reactor used for the Ea test is the same as that used for the activity evaluation. The appropriate amount of catalysts diluted with SiO2 powder were used in the kinetics experiments. During kinetics tests, the CO2 conversion is controlled below 15% by adjusting the temperature and gas flow rate. The reaction orders of CO2 and H2 for the catalysts were collected under 250, 280, and 300 °C. The RWGS activity was recorded while the concentration of CO2 or H2 in the reaction gas was varied on purpose.
Theoretical calculations
Details of the computational methods and the simulation model are put in the Supplementary Information.
Data availability
The main data supporting the findings of this study are available within the article and its Supplementary Information. Extra data are available from the corresponding author upon request. Source data are provided with this paper.
References
Christopher, B. M. et al. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 341, 771–773 (2013).
Lee, S. et al. A measure of active interfaces in supported catalysts for high-temperature reactions. Chem 8, 815–835 (2022).
Ma, D. et al. Maximizing the synergistic effect of CoNi catalyst on α‑MoC for robust hydrogen production. J. Am. Chem. Soc. 143, 628–633 (2021).
Heyden, A. et al. On the importance of metal-oxide interface sites for the water–gas shift reaction over Pt/CeO2 catalysts. J. Catal. 309, 314–324 (2014).
Li, J. et al. In situ Raman observation of oxygen activation and reaction at platinum–ceria interfaces during CO oxidation. J. Am. Chem. Soc. 143, 15635–15643 (2021).
Li, Y. et al. Dynamic structure of active sites in ceria-supported Pt catalysts for the water gas shift reaction. Nat. Commun. 12, 914 (2021).
Christopher, W. J. et al. Synergy between ceria oxygen vacancies and Cu nanoparticles facilitates the catalytic conversion of CO2 to CO under mild conditions. ACS Catal. 8, 12056–12066 (2018).
Huang, W. et al. Structure sensitivity of Au–TiO2 strong metal–support interactions. Angew. Chem. Int. Ed. 60, 12074–12081 (2021).
Rodriguez, J. A. et al. Exploring metal–support interactions to immobilize subnanometer Co clusters on γ−Mo2N: A highly selective and stable catalyst for CO2 activation. ACS Catal. 9, 9087–9097 (2019).
Christopher, P. et al. Adsorbate-mediated strong metal–support interactions in oxide-supported Rh catalysts. Nat. Chem. 9, 120–127 (2017).
Wang, S. et al. Defect chemistry in heterogeneous catalysis: Recognition, understanding, and utilization. ACS Catal. 10, 11082–11098 (2020).
Wang, Y. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Bobadilla, L. F. et al. Unravelling the role of oxygen vacancies in the mechanism of the reverse water–gas shift reaction by operando DRIFTS and ultraviolet–visible spectroscopy. ACS Catal. 8, 7455–7467 (2018).
Zeng, J. et al. Oxygen vacancies in ZnO nanosheets enhance CO2 electrochemical reduction to CO. Angew. Chem. Int. Ed. 57, 6054–6059 (2018).
Nelson, N. C., Chen, L., Meira, D., Kovarik, L. & Szanyi, J. In situ dispersion of palladium on TiO2 during reverse water–gas shift reaction: Formation of atomically dispersed palladium. Angew. Chem. Int. Ed. 59, 17657–17663 (2020).
Fu, X. et al. Direct identification of active surface species for the water–gas shift reaction on a gold-ceria catalyst. J. Am. Chem. Soc. 141, 4613–4623 (2019).
Chen, A. et al. Structure of the catalytically active copper-ceria interfacial perimeter. Nat. Catal. 2, 334–341 (2019).
Rodriguez, J. A. et al. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: Morphological effects of nanostructured ceria on the catalytic activity. J. Phys. Chem. C 122, 12934–12943 (2018).
Yu, W. et al. Very high loading oxidized copper supported on ceria to catalyze the water–gas shift reaction. J. Catal. 402, 83–93 (2021).
Liu, X. et al. Insights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal. 10, 11493–11503 (2020).
Zhang, Z. et al. Intrinsically active surface in a Pt/γ-Mo2N catalyst for the water–gas shift reaction: Molybdenum nitride or molybdenum oxide? J. Am. Chem. Soc. 142, 13362–13371 (2020).
Bahmanpour, A. M. et al. Cu–Al spinel as a highly active and stable catalyst for the reverse water gas shift reaction. ACS Catal. 9, 6243–6251 (2019).
Sun, J. et al. Ultra-high thermal stability of sputtering reconstructed Cu-based catalysts. Nat. Commun. 12, 7209 (2021).
Ma, D. et al. Reversing sintering effect of Ni particles on γ-Mo2N via strong metal support interaction. Nat. Commun. 12, 6978 (2021).
Christoph, R. M. et al. Two-dimensional molybdenum carbide 2D-Mo2C as a superior catalyst for CO2 hydrogenation. Nat. Commun. 12, 5515 (2021).
Williamson, D. L., Herdes, C., Torrente-Murciano, L., Jones, M. D. & Mattia, D. N-doped Fe@CNT for combined RWGS/FT CO2 hydrogenation. ACS Sustain. Chem. Eng. 7, 7395–7402 (2019).
Fu, X. et al. Co3O4–Al2O3 mesoporous hollow spheres as efficient catalyst for Fischer–Tropsch synthesis. Appl. Catal. B: Environ. 211, 176–187 (2017).
Ma, D. et al. Development of direct conversion of syngas to unsaturated hydrocarbons based on Fischer–Tropsch route. Chem 7, 3027–3051 (2021).
Fu, H. et al. Synergism of molybdenum nitride and palladium for high-efficiency formic acid electrooxidation. J. Mater. Chem. A 6, 7623–7630 (2018).
Zeng, J. et al. Symmetry-breaking sites for activating linear carbon dioxide molecules. Acc. Chem. Res. 54, 1454–1464 (2021).
Álvarez, A. et al. CO2 activation over catalytic surfaces. ChemPhysChem 18, 3135–3141 (2017).
Su, X., Yang, X., Zhao, B. & Huang, Y. Designing of highly selective and high-temperature endurable RWGS heterogeneous catalysts: Recent advances and the future directions. J. Energy Chem. 26, 854–867 (2017).
Chen, X. et al. Identification of relevant active sites and a mechanism study for reverse water gas shift reaction over Pt/CeO2 catalysts. J. Energy Chem. 25, 1051–1057 (2016).
Zhang, X. et al. Synergy between β-Mo2C nanorods and non-thermal plasma for selective CO2 reduction to CO. Chem 6, 3312–3328 (2020).
Wang, W. et al. Highly dispersed copper oxide clusters as active species in copper-ceria catalyst for preferential oxidation of carbon monoxide. ACS Catal. 5, 2088–2099 (2015).
Ma, D. et al. Highly dispersed copper over β-Mo2C as an efficient and stable catalyst for the reverse water gas shift (RWGS) reaction. ACS Catal. 7, 912–918 (2017).
Xie, Z. et al. Atomically dispersed Pt/CeO2 catalyst with superior CO selectivity in reverse water gas shift reaction. Appl. Catal. B: Environ. 291, 120101 (2021).
Dumesic, J. A. et al. Reverse water–gas shift on interfacial sites formed by deposition of oxidized molybdenum moieties onto gold nanoparticles. J. Am. Chem. Soc. 137, 10317–10325 (2015).
Wang, W. et al. Reverse water gas shift over In2O3–CeO2 catalysts. Catal. Today 259, 402–408 (2016).
Liu, W. et al. Reversible loss of core–shell structure for Ni–Au bimetallic nanoparticles during CO2 hydrogenation. Nat. Catal. 3, 411–417 (2020).
Burch, R. et al. Spectrokinetic investigation of reverse water–gas-shift reaction intermediates over a Pt/CeO2 catalyst. J. Phys. Chem. B 108, 20240–20246 (2004).
Yu, Y. et al. Highly active and stable copper catalysts derived from copper silicate double-shell nanofibers with strong metal–support interactions for the RWGS reaction. Chem. Commun. 55, 4178–4181 (2019).
Chen, X. et al. Catalytic performance of the Pt/TiO2 catalysts in reverse water gas shift reaction: Controlled product selectivity and a mechanism study. Catal. Today 281, 312–318 (2017).
Xiao, F. et al. Product selectivity controlled by nanoporous environments in zeolite crystals enveloping rhodium nanoparticle catalysts for CO2 hydrogenation. J. Am. Chem. Soc. 141, 8482–8488 (2019).
Xiao, F. et al. Dispersed nickel boosts catalysis by copper in CO2 hydrogenation. ACS Catal. 10, 9261–9270 (2020).
Zhang, T. et al. Promotion effects of potassium on the activity and selectivity of Pt/zeolite catalysts for reverse water gas shift reaction. Appl. Catal. B: Environ. 216, 95–105 (2017).
Acknowledgements
This work was financially supported from the National Key Basic Research Program of China (2021YFA1501102, 2021YFA1501103), the National Science Foundation of China (no. 21803036, 22075166, 21725301, 21932002), the Taishan Scholar Project of Shandong Province of China, the CAS Project for Young Scientists in Basic Research (YSBR-054), and the Young Scholars Program of Shandong University (2018WLJH49). We thank the Center of Structural Characterizations and Property Measurements at Shandong University for the help on sample characterizations. D.M. acknowledges support from the Tencent Foundation through the XPLORER PRIZE.
Author information
Authors and Affiliations
Contributions
C.J.J. and D.M. supervised the work; H.X.L., C.J.J., and D.M. designed the experiments, analyzed the results, and wrote the manuscript; J.Y.L. and Q.F. carried out the first-principles simulations; X.T.Q performed the XAFS measurements. H.X.L., K.X., and W.W.W. performed the in situ DRIFTS, in situ Raman, and Quasi in situ XPS; H.X.L., K.X., and H.Y. performed the catalysts preparation, catalytic tests, and the TPR tests; C.M. performed the aberration-corrected HAADF-STEM measurements and analyzed the results. D.Q.X. gave a lot of suggestions and helped to modify the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Communications thanks Luis F. Bobadilla and the other, anonymous, reviewer for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Source data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, HX., Li, JY., Qin, X. et al. Ptn–Ov synergistic sites on MoOx/γ-Mo2N heterostructure for low-temperature reverse water–gas shift reaction. Nat Commun 13, 5800 (2022). https://doi.org/10.1038/s41467-022-33308-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41467-022-33308-7
This article is cited by
-
Reverse water gas-shift reaction product driven dynamic activation of molybdenum nitride catalyst surface
Nature Communications (2024)
-
Redox-induced controllable engineering of MnO2-MnxCo3-xO4 interface to boost catalytic oxidation of ethane
Nature Communications (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.